Colorimetric and Electrochemical Dual-Mode Detection of Thioredoxin 1 Based on the Efficient Peroxidase-Mimicking and Electrocatalytic Property of Prussian Blue Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Characterization of PBNPs
2.3. Evaluation of Peroxidase-Mimicking and Electrocatalytic Activity of PBNPs
2.4. Preparation of PBNPs Conjugated with TRX1 Antibody (PBNPs-Ab)
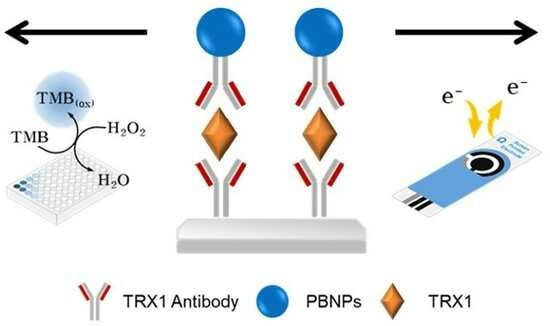
2.5. Dual-Mode Immunoassay for TRX1 Utilizing Peroxidase-like and Electrocatalytic Activities of PBNPs
3. Results and Discussion
3.1. Synthesis and Characterization of PBNPs
3.2. Investigation of Peroxidase-like and Electrocatalytic Activity of PBNPs
3.3. PBNPs-Based Colorimetric and Electrochemical Dual-Mode Immunoassay for TRX1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.-M.; Li, Y.; Wang, X.; Cao, W.-M.; Liu, D.-X. Non-invasive biomarkers for early detection of breast cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Esteller, M.; Chatzaki, E. Circulating cell-free dna in breast cancer: Searching for hidden information towards precision medicine. Cancers 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Chu, P.-Y. Current and developing liquid biopsy techniques for breast cancer. Cancers 2022, 14, 2052. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, S.; Daryani, S.; Eslami-S, Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Majidzadeh-A, K.; Esmaeili, R. Exosomes in cancer liquid biopsy: A focus on breast cancer. Mol. Ther. Nucleic Acids 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef]
- Cha, M.-K.; Suh, K.-H.; Kim, I.-H. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 93. [Google Scholar] [CrossRef]
- Molina, R.; Jo, J.; Filella, X.; Zanon, G.; Pahisa, J.; Muñoz, M.; Farrus, B.; Latre, M.L.; Escriche, C.; Estape, J.; et al. c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: Prognostic value. Breast Cancer Res. Treat. 1998, 51, 109–119. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and related molecules–from biology to health and disease. Antioxid. Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; McGrath, K.L.; Di Trapani, G.; Charoentong, P.; Shah, F.; King, M.M.; Clarke, F.M.; Tonissen, K.F. The thioredoxin system in breast cancer cell invasion and migration. Redox Biol. 2016, 8, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-J.; Geng, W.-S.; Wang, Z.-Q.; Chen, L.; Zeng, X.-S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.; Mustafa, S.M. Thioredoxin system: A model for determining novel lead molecules for breast cancer chemotherapy. Avicenna J. Med. Biotechnol. 2012, 4, 121–130. [Google Scholar] [PubMed]
- Park, B.-J.; Cha, M.-K.; Kim, I.-H. Thioredoxin 1 as a serum marker for breast cancer and its use in combination with CEA or CA15-3 for improving the sensitivity of breast cancer diagnoses. BMC Res. Notes 2014, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Nakashima, T.; Yoh, T.; Nakajima, Y.; Ishikawa, H.; Mitsuyoshi, H.; Sakamoto, Y.; Okanoue, T.; Kashima, K.; Nakamura, H.; et al. Serum thioredoxin levels as an indicator of oxidative stress in patients with hepatitis C virus infection. J. Hepatol. 2000, 33, 616–622. [Google Scholar] [CrossRef]
- Kishimoto, C.; Shioji, K.; Nakamura, H.; Nakayama, Y.; Yodoi, J.; Sasayama, S. Serum thioredoxin (TRX) levels in patients with heart failure. Jpn. Circ. J. 2001, 65, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Zhang, J.; Xu, N.; Liu, B.; Li, M.; Liu, A.; Li, A.; Tang, H. Diagnostic value analysis of combined detection of Trx, CYFRA21-1 and SCCA in lung cancer. Oncol. Lett. 2019, 17, 4293–4298. [Google Scholar] [CrossRef] [PubMed]
- Farka, Z.K.; Čunderlová, V.; Horáčková, V.; Pastucha, M.J.; Mikušová, Z.; Hlaváček, A.N.; Skládal, P. Prussian blue nanoparticles as a catalytic label in a sandwich nanozyme-linked immunosorbent assay. Anal. Chem. 2018, 90, 2348–2354. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, L.; Tang, Q.; Li, H.; Zhang, L.; Wei, Q. A nanozyme-linked immunosorbent assay for dual-modal colorimetric and ratiometric fluorescent detection of cardiac troponin I. Sens. Actuators B Chem. 2019, 288, 60–64. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, P.T.; Le, T.N.; Kim, M.I. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens. Bioelectron. 2021, 182, 113187. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.B.; Shiddiky, M.J. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review—Nanozyme-based immunosensors and immunoassays: Recent developments and future trends. J. Electrochem. Soc. 2020, 167, 037508. [Google Scholar] [CrossRef]
- Di, H.; Mi, Z.; Sun, Y.; Liu, X.; Liu, X.; Li, A.; Jiang, Y.; Gao, H.; Rong, P.; Liu, D. Nanozyme-assisted sensitive profiling of exosomal proteins for rapid cancer diagnosis. Theranostics 2020, 10, 9303. [Google Scholar] [CrossRef]
- Woo, M.A.; Kim, M.I.; Jung, J.H.; Park, K.S.; Seo, T.S.; Park, H.G. A novel colorimetric immunoassay utilizing the peroxidase mimicking activity of magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 9999–10014. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.Y.; Heo, N.S.; Kang, J.W.; Lee, J.B.; Kim, H.J.; Kim, M.I. Nanoceria-based lateral flow immunoassay for hydrogen peroxide-free colorimetric biosensing for C-reactive protein. Anal. Bioanal. Chem. 2022, 414, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Paolella, A.; Faure, C.; Timoshevskii, V.; Marras, S.; Bertoni, G.; Guerfi, A.; Vijh, A.; Armand, M.; Zaghib, K. A review on hexacyanoferrate-based materials for energy storage and smart windows: Challenges and perspectives. J. Mater. Chem. A 2017, 5, 18919–18932. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Prussian blue: A safe pigment with zeolitic-like activity. Int. J. Mol. Sci. 2021, 22, 780. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Mo, Z.; Shuai, C.; He, S.; Liu, W.; Liu, G.; Du, Y.; Dong, Q.; Ding, J.; Zhu, X.; et al. N-doped bimetallic NiFeP nanocubic clusters derived from Prussian blue analogues as a high-efficiency and durable water splitting electrocatalyst. J. Electroanal. Chem. 2022, 918, 116427. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically synthesized prussian blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Liu, Y.; Jin, W. Recent progress in Prussian blue films: Methods used to control regular nanostructures for electrochemical biosensing applications. Biosens. Bioelectron. 2017, 96, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Hamidi, H.; Gorton, L. Electrochemical behavior and application of Prussian blue nanoparticle modified graphite electrode. Sens. Actuators B Chem. 2010, 147, 270–276. [Google Scholar] [CrossRef]
- Yamamoto, T.; Saso, N.; Umemura, Y.; Einaga, Y. Photoreduction of prussian blue intercalated into titania nanosheet ultrathin films. J. Am. Chem. Soc. 2009, 131, 13196–13197. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.A.; Estelrich, J. Prussian blue nanoparticles: Synthesis, surface modification, and biomedical applications. Drug Discov. Today 2020, 25, 1431–1443. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Cai, X.; Gao, W.; Tang, X.; Chen, Y.; Chen, J.; Chen, L.; Tian, Q.; Yang, S.; et al. Large-scale synthesis of monodisperse Prussian blue nanoparticles for cancer theranostics via an “in situ modification” strategy. Int. J. Nanomed. 2018, 14, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Torad, N.L.; Chiang, Y.D.; Wu, K.C.W.; Yamauchi, Y. Size-and shape-controlled synthesis of Prussian Blue nanoparticles by a polyvinylpyrrolidone-assisted crystallization process. Crystengcomm 2012, 14, 3387–3396. [Google Scholar] [CrossRef]
- Huang, C.; Wen, T.; Shi, F.J.; Zeng, X.Y.; Jiao, Y.J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. The electrocatalytic activity of Prussian blue in hydrogen peroxide reduction studied using a wall-jet electrode with continuous flow. J. Electroanal. Chem. 1998, 456, 97–104. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.; Li, C.-C.; Wang, C.-M.; Zhang, C.-Y. An electrochemical biosensor based on the enhanced quasi-reversible redox signal of prussian blue generated by self-sacrificial label of iron metal-organic framework. Biosens. Bioelectron. 2018, 122, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, M.; Torrente-Rodríguez, R.M.; Kozell, A.; Liao, W.C.; Cecconello, A.; Campuzano, S.; Pingarrón, J.M.; Willner, I. Mimicking peroxidase activities with prussian blue nanoparticles and their cyanometalate structural analogues. Nano Lett. 2017, 17, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, D.; Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 2014, 120, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Zhou, Y.; Xian, Y.; Zhou, L.; Wang, H.; Jin, L. Preparation of poly (vinylpyrrolidone)-protected Prussian blue nanoparticles-modified electrode and its electrocatalytic reduction for hemoglobin. Anal. Chim. Acta 2005, 546, 139–146. [Google Scholar] [CrossRef]
- Maeng, H.J.; Kim, D.-H.; Kim, N.-W.; Ruh, H.; Lee, D.K.; Yu, H. Synthesis of spherical Prussian blue with high surface area using acid etching. Curr. Appl. Phys. 2018, 18, S21–S27. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, M.; Wang, Y.Q.; Zhang, Q.; Zhou, H.; Zhao, H.L. Electrochromic properties of three-color prussian green ECDs and the evolution of Fe ions during the discoloration process. Mater. Lett. 2022, 311, 131627. [Google Scholar] [CrossRef]
- Nawar, A.M.; Alzharani, A.A. Impedance spectroscopy and conduction mechanism analysis of bulk nanostructure Prussian blue pellets. Mater. Chem. Phys. 2023, 306, 128000. [Google Scholar] [CrossRef]
- Isfahani, V.B.; Memarian, N.; Dizaji, H.R.; Arab, A.; Silva, M.M. The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, E.S.; Kim, T.H.; Jeon, J.W.; Kim, Y.; Oh, B.K. Detection of thioredoxin-1 using ultra-sensitive ELISA with enzyme-encapsulated human serum albumin nanoparticle. Nano Converg. 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Jampaiah, D.; Reddy, T.S.; Kandjani, A.E.; Selvakannan, P.; Sabri, Y.M.; Coyle, V.E.; Shukla, R.; Bhargava, S.K. Fe-doped CeO2 nanorods for enhanced peroxidase-like activity and their application towards glucose detection. J. Mat. Chem. B 2016, 4, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiao, L.; Yan, H.; Wu, Y.; Chen, L.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Glucose oxidase-integrated metal–organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing. ACS Appl. Mater. Interfaces 2019, 11, 22096–22101. [Google Scholar] [CrossRef]
- Chen, J.; Gao, H.; Li, Z.; Li, Y.; Yuan, Q. Ferriporphyrin-inspired MOFs as an artificial metalloenzyme for highly sensitive detection of H2O2 and glucose. Chin. Chem. Lett. 2020, 31, 1398–1401. [Google Scholar] [CrossRef]
- Zhu, Z.; Gong, L.; Miao, X.; Chen, C.; Su, S. Prussian Blue Nanoparticle Supported MoS2 Nanocomposites as a Peroxidase-like Nanozyme for Colorimetric Sensing of Dopamine. Biosensors 2022, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Jiang, X.; Yang, M.; Wang, X. Sensitive colorimetric assay for the determination of alkaline phosphatase activity utilizing nanozyme based on copper nanoparticle-modified Prussian blue. Anal. Bioanal. Chem. 2021, 413, 3955–3963. [Google Scholar] [CrossRef] [PubMed]





| Detection Mode | Spiked Level (ng mL−1) | Measured (ng mL−1) a | SD b | Recovery (%) c | CV (%) d |
|---|---|---|---|---|---|
| Colorimetric method | 10 | 10.57 | 0.73 | 105.70 | 6.96 |
| 25 | 26.48 | 1.65 | 105.92 | 6.25 | |
| 50 | 49.82 | 1.01 | 99.64 | 2.53 | |
| Electrochemical method | 10 | 9.93 | 0.29 | 99.26 | 2.92 |
| 25 | 24.53 | 0.69 | 98.13 | 3.53 | |
| 50 | 46.22 | 2.96 | 92.44 | 6.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.U.; Kim, J.M.; Thamilselvan, A.; Nam, K.-H.; Kim, M.I. Colorimetric and Electrochemical Dual-Mode Detection of Thioredoxin 1 Based on the Efficient Peroxidase-Mimicking and Electrocatalytic Property of Prussian Blue Nanoparticles. Biosensors 2024, 14, 185. https://doi.org/10.3390/bios14040185
Kim JU, Kim JM, Thamilselvan A, Nam K-H, Kim MI. Colorimetric and Electrochemical Dual-Mode Detection of Thioredoxin 1 Based on the Efficient Peroxidase-Mimicking and Electrocatalytic Property of Prussian Blue Nanoparticles. Biosensors. 2024; 14(4):185. https://doi.org/10.3390/bios14040185
Chicago/Turabian StyleKim, Jeong Un, Jee Min Kim, Annadurai Thamilselvan, Ki-Hwan Nam, and Moon Il Kim. 2024. "Colorimetric and Electrochemical Dual-Mode Detection of Thioredoxin 1 Based on the Efficient Peroxidase-Mimicking and Electrocatalytic Property of Prussian Blue Nanoparticles" Biosensors 14, no. 4: 185. https://doi.org/10.3390/bios14040185
APA StyleKim, J. U., Kim, J. M., Thamilselvan, A., Nam, K. -H., & Kim, M. I. (2024). Colorimetric and Electrochemical Dual-Mode Detection of Thioredoxin 1 Based on the Efficient Peroxidase-Mimicking and Electrocatalytic Property of Prussian Blue Nanoparticles. Biosensors, 14(4), 185. https://doi.org/10.3390/bios14040185