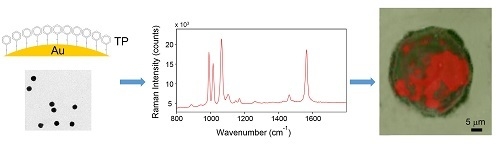
Functionalized Gold Nanoparticles as Biosensors for Monitoring Cellular Uptake and Localization in Normal and Tumor Prostatic Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Gold Nanoparticles
2.2.1. Preparation of Gold Seeds Solution
2.2.2. Preparation of Growth Solution
2.2.3. Seeding Growth
2.2.4. Functionalization of Gold Nanoparticles
2.3. Cell Culture and Cytotoxicity Assay
2.4. Nanoparticles Uptake
2.5. Techniques
2.5.1. Transmission Electron Microscopy (TEM)
2.5.2. Statistical Image Analysis (SIA)
2.5.3. UV-VIS Spectroscopy
2.5.4. Dynamic Light Scattering (DLS) and Zeta-Potential Measurements
2.5.5. Raman Spectroscopy
3. Results and Discussion
3.1. Nanoparticle Characterization
3.1.1. TEM/SIA
3.1.2. DLS and Z-Potential Measurements
3.1.3. UV-VIS Absorption Spectroscopy
3.1.4. SERS
3.2. Nanoparticles Cytotoxicity
3.3. Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.; Panwar, N.; Yap, S.H.K.; Wu, Q.; Zeng, S.; Xu, J.; Tjin, S.C.; Song, J.; Qu, J.; Yong, K.-T. SERS-based ultrasensitive sensing platform: An insight into design and practical applications. Coord. Chem. Rev. 2017, 337, 1–33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.-I.; Sharma, B.; Cardinal, M.F.; Kurouski, D.; van Duyne, R.P. Surface-enhanced raman spectroscopy biosensing: In vivo diagnostics and multimodal imaging. Anal. Chem. 2016, 88, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Wang, H.-N.; Scaffidi, J. Plasmonic nanoprobes for SERS biosensing and bioimaging. J. Biophotonics 2010, 3, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Fales, A.M.; Griffin, G.D.; Khoury, C.G.; Liu, Y.; Ngo, H.; Norton, S.J.; Register, J.K.; Wang, H.-N.; Yuan, H. Plasmonic nanoprobes: From chemical sensing to medical diagnostics and therapy. Nanoscale 2013, 5, 10127–10140. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, S.H.; Gan, C.W.; Feng, S.-S. In vitro and in vivo investigation on PLA–TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm. Res. 2008, 25, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Jiang, H.; Hochwald, S.N.; Delano, M.; Cance, W.G.; Grobmyer, S.R. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 2006, 107, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Yih, T.C.; Wei, C. Nanomedicine in cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding Growth for Size Control of 5–40 nm Diameter Gold Nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Ziegler, C.; Eychmüller, A. Seeded Growth Synthesis of Uniform Gold Nanoparticles with Diameters of 15–300 nm. J. Phys. Chem. C 2011, 115, 4502–4506. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Jackson, J.B.; Halas, N.J. Surface-enhanced Raman scattering on tunable plasmonic nanoparticle substrates. Proc. Natl. Acad. Sci. USA 2004, 101, 17930–17935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Fu, C.; Wang, H.; Xu, S.; Xu, W. Aptamer-based surface-enhanced Raman scattering (SERS) sensor for thrombin based on supramolecular recognition, oriented assembly, and local field coupling. Anal. Bioanal. Chem. 2017, 409, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced Raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Jiao, A.; Yang, R.; Li, H.; Li, J.; Shi, M.; Ma, C.; Jiang, Y.; Deng, L.; Tan, W. Fabricating a reversible and regenerable raman-active substrate with a biomolecule-controlled DNA nanomachine. J. Am. Chem. Soc. 2012, 134, 19957–19960. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Vasudev, M.; Dutta, M.; Stroscio, M.A. Raman and Surface-Enhanced Raman Scattering (SERS) Studies of the Thrombin-Binding Aptamer. IEEE Trans. NanoBiosci. 2013, 12, 93–97. [Google Scholar]
- Kim, N.H.; Lee, S.J.; Moskovits, M. Aptamer-Mediated Surface-Enhanced Raman Spectroscopy Intensity Amplification. Nano Lett. 2010, 10, 4181–4185. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Calarco, A.; Pannico, M.; la Manna, P.; Margarucci, S.; Tafuri, A.; Peluso, G. Hyperspectral Raman imaging of human prostatic cells: An attempt to differentiate normal and malignant cell lines by univariate and multivariate data analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Gregas, M.K.; Scaffidi, J.P.; Lauly, B.; Vo-Dinh, T. Surface-Enhanced Raman Scattering Detection and Tracking of Nanoprobes: Enhanced Uptake and Nuclear Targeting in Single Cells. Appl. Spectrosc. 2010, 64, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Gold Nanoparticles with a polymerizable surfactant bilayer: Synthesis, polymerization, and stability evaluation. Langmuir 2009, 25, 13874–13879. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, D.; Xu, X.; Gu, R. Theoretical and experimental studies on the adsorption behavior of thiophenol on gold nanoparticles. J. Raman Spectrosc. 2007, 38, 1436–1443. [Google Scholar] [CrossRef]
- Das, M.; Mordoukhovski, L.; Kumacheva, E. Sequestering gold nanorods by polymer microgels. Adv. Mater. 2008, 20, 2371–2375. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Evidence for bilayer assembly of cationic surfactants on the surface of gold nanorods. Langmuir 2001, 17, 6368–6374. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Self-assembly patterns formed upon solvent evaporation of aqueous cetyltrimethylammonium bromide-coated gold nanoparticles of various shapes. Langmuir 2005, 21, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV−vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Ziomek, S.J. Introductory Group Theory and Its Application to Molecular Structure; Plenum Press: New York, NY, USA, 1975. [Google Scholar]
- Alsharif, S.A.; Chen, L.Y.; Tlahuice-Flores, A.; Whetten, R.L.; Yacaman, M.J. Interaction between functionalized gold nanoparticles in physiological saline. Phys. Chem. Chem. Phys. 2014, 16, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, L.; Kang, Y.; He, Z.; Xiong, F.; Ling, X.; Wu, J. Significant suppression of non-small-cell lung cancer by hydrophobic poly(ester amide) nanoparticles with high docetaxel loading. Front. Pharmacol. 2018, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.S.; Mukherjee, P. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]










| Raman (cm−1) | SERS (cm−1) | Symmetry | Assignments [29] |
|---|---|---|---|
| 920 | ─ | ─ | S─H in-plane bending |
| 1003 | 1002 | a1 | Ring out-of-plane deformation/C–H out-of-plane bending |
| 1028 | 1027 | a1 | Ring in-plane deformation/C–C symmetric stretching |
| 1096 | 1078 | b1 | C–C anti-symmetric stretching |
| 1122 | 1117 | ─ | ─ |
| 1161 | 1159 | b1 | C–H in-plane bending |
| 1184 | 1182 | a1 | C–H in-plane bending |
| 1587 | 1576 | b1 | C–C symmetric stretching |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannico, M.; Calarco, A.; Peluso, G.; Musto, P. Functionalized Gold Nanoparticles as Biosensors for Monitoring Cellular Uptake and Localization in Normal and Tumor Prostatic Cells. Biosensors 2018, 8, 87. https://doi.org/10.3390/bios8040087
Pannico M, Calarco A, Peluso G, Musto P. Functionalized Gold Nanoparticles as Biosensors for Monitoring Cellular Uptake and Localization in Normal and Tumor Prostatic Cells. Biosensors. 2018; 8(4):87. https://doi.org/10.3390/bios8040087
Chicago/Turabian StylePannico, Marianna, Anna Calarco, Gianfranco Peluso, and Pellegrino Musto. 2018. "Functionalized Gold Nanoparticles as Biosensors for Monitoring Cellular Uptake and Localization in Normal and Tumor Prostatic Cells" Biosensors 8, no. 4: 87. https://doi.org/10.3390/bios8040087
APA StylePannico, M., Calarco, A., Peluso, G., & Musto, P. (2018). Functionalized Gold Nanoparticles as Biosensors for Monitoring Cellular Uptake and Localization in Normal and Tumor Prostatic Cells. Biosensors, 8(4), 87. https://doi.org/10.3390/bios8040087