Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals
2.3. Preparation of the Pt/PPD/GOx biosensor
2.4. Electrochemical Estimation of Heavy Metal Ions
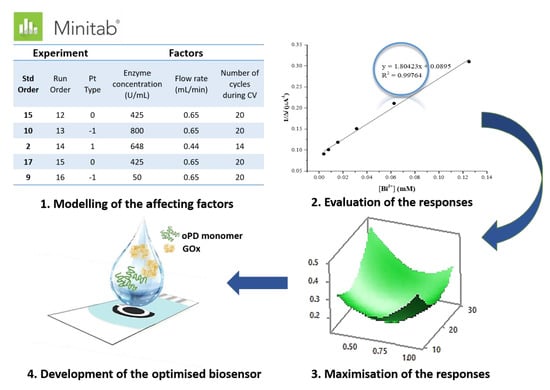
2.5. Experimental Design
3. Results and Discussion
3.1. Glucose Responses and Inhibitive Detection of Heavy Metal Ions in A Fia Apparatus
3.2. Optimisation of the Performance of Biosensor Using DOE
3.3. Analytical Performances of the Optimised Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.; Lin, C.; He, M.; Hao, F.; Liu, H.; Zhu, W. Heavy metal loss from agricultural watershed to aquatic system: A scientometrics review. Sci. Total Environ. 2018, 637–638, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, M.W. Molecules and elements for quantitative bioanalysis: The allure of using electrospray, MALDI, and ICP mass spectrometry side-by-side. Mass Spectrom. Rev. 2018, 38(2), 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; De Alda, M.J.L.; Marco, M.P.; Barceló, D. Biosensors for environmental monitoring: A global perspective. Talanta 2005, 65, 291–297. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Bachan Upadhyay, L.S.; Verma, N. Enzyme Inhibition Based Biosensors: A Review. Anal. Lett. 2012, 46, 225–241. [Google Scholar] [CrossRef]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose Fast-Response Amperometric Sensor Based on Glucose Oxidase Immobilized in an Electropolymerized Poly(o-phenylenediamine) Film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Mikoliunaite, L.; Gicevičius, M.; Popov, A.; Ramanavičiene, A. Conducting polymers in the design of enzymatic sensors. In Proceedings of the IEEE 7th International Conference on Nanomaterials: Applications and Properties (NAP), Odessa, Ukraine, 10–15 September 2017. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. Annu. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef]
- Bolella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electroc. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Mugheri, A.Q.; Tahira, A.; Sirajuddin; Sherazi, S.T.H.; Ishaque Abro, M.; Willander, M.; Ibupoto, Z.H. An amperometric indirect determination of heavy metal ions through inhibition of glucose oxidase immobilized on cobalt oxide nanostructures. Sens. Lett. 2016, 14. [Google Scholar] [CrossRef]
- Rust, I.M.; Goran, J.M.; Stevenson, K.J. Amperometric Detection of Aqueous Silver Ions by Inhibition of Glucose Oxidase Immobilized on Nitrogen-Doped Carbon Nanotube Electrodes. Anal. Chem. 2015, 87, 7250–7257. [Google Scholar] [CrossRef] [PubMed]
- Ayenimo, J.G.; Adeloju, S.B. Inhibitive potentiometric detection of trace metals with ultrathin polypyrrole glucose oxidase biosensor. Talanta 2015, 137, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.E.; Carvalho, R.C.; Amine, A.; Brett, C.M.A. Glucose oxidase enzyme inhibition sensors for heavy metals at carbon film electrodes modified with cobalt or copper hexacyanoferrate. Sens. Actuators B Chem. 2013, 178, 270–278. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Glucose oxidase inhibition in poly(neutral red) mediated enzyme biosensors for heavy metal determination. Microchim. Acta. 2008, 163, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Amine, A.; Mohammadi, H.; Bourais, I.; Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2016, 76, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. Biosensors and Bioelectronics A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Herrera, M.C. Enzyme inhibition-based biosensors and biosensing systems: Questionable analytical devices. Biosens. Bioelectron. 2003, 18, 279–294. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, S.K.; Bhardwaj, N.; Paul, A.K.; Kumar, P.; Kim, K.H.; Deep, A. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol. Adv. 2016, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Nurani, S.; Abdul, H.; Lau, W. Chemical Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications. Sens. Actuators B. Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Tarley, C.R.T.; Silveira, G.; dos Santos, W.N.L.; Matos, G.D.; da Silva, E.G.P.; Bezerra, M.A.; Miró, M.; Ferreira, S.L.C. Chemometric tools in electroanalytical chemistry: Methods for optimization based on factorial design and response surface methodology. Microchem. J. 2009, 92, 58–67. [Google Scholar] [CrossRef]
- Vera, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, C. Talanta Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Monaci, L.; Vatinno, R.; De Benedetto, G.E. Fast detection of cyclopiazonic acid in cheese using Fourier transform mid-infrared ATR spectroscopy. Eur. Food. Res. Technol. 2007, 225, 585–588. [Google Scholar] [CrossRef]
- Mignani, A.; Luciano, G.; Lanteri, S.; Leardi, R.; Scavetta, E.; Tonelli, D. Optimization of a glucose biosensor setup based on a Ni/Al HT matrix. Anal. Chim. Acta. 2007, 599, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Talat, M.; Hasan, S.H.; Pandey, R.K. Factorial design for the optimization of enzymatic detection of cadmium in aqueous solution using immobilized urease from vegetable waste. Bioresour. Technol. 2008, 99, 7565–7572. [Google Scholar] [CrossRef]
- Uliana, C.V.; Tognolli, J.O.; Yamanaka, H. Application of factorial design experiments to the development of a disposable amperometric DNA biosensor. Electroanalysis 2011, 23, 2607–2615. [Google Scholar] [CrossRef]
- Horry, H.; Maul, A. Optimization of a bacterial bioluminescent biosensor through experimental design. Sens. Actuators B. Chem. 2007, 127, 649–657. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Shojaosadati, S.A.; Ranaie, S.O.; Mousavi, S.M. Optimization and evaluation of acetylcholine esterase immobilization on ceramic packing using response surface methodology. Process. Biochem. 2010, 45, 81–87. [Google Scholar] [CrossRef]
- Malitesta, C.; Guascito, M.R. Heavy metal determination by biosensors based on enzyme immobilised by electropolymerisation. Biosens. Bioelectron. 2005, 20, 1643–1647. [Google Scholar] [CrossRef]
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor. Study of the effect of hydrogen peroxide decomposition. Sens. Actuators B Chem. 2008, 131, 394–402. [Google Scholar] [CrossRef]
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Screen-Printed Glucose Oxidase-Based Biosensor for Inhibitive Detection of Heavy Metal Ions in a Flow Injection System. Sens. Lett. 2009, 7, 153–159. [Google Scholar] [CrossRef]
- Yang, Q.; Qu, Y.; Bo, Y.; Wen, Y.; Huang, S. Biosensor for atrazin based on aligned carbon nanotubes modified with glucose oxidase. Microchim. Acta. 2010, 168, 197–203. [Google Scholar] [CrossRef]






| Experiment | Factors | Responses (Sensitivity, µA·mM−1) | |||||
|---|---|---|---|---|---|---|---|
| RdesStdOrder | RunOrder | PtType | Enzyme Concentration (U/mL) | Flow Rate (mL/min) | Number of Cycles during CV | Al3+ | Bi3+ |
| 3 | 1 | 1 | 202 | 0.86 | 14 | 0.068 | 1.059 |
| 12 | 2 | −1 | 425 | 1 | 20 | 0.035 | 0.657 |
| 14 | 3 | −1 | 425 | 0.65 | 30 | 0.026 | 0.509 |
| 20 | 4 | 0 | 425 | 0.65 | 20 | 0.060 | 0.438 |
| 19 | 5 | 0 | 425 | 0.65 | 20 | 0.025 | 0.454 |
| 11 | 6 | −1 | 425 | 0.3 | 20 | 0.077 | 0.599 |
| 5 | 7 | 1 | 202 | 0.44 | 26 | 0.417 | 1.675 |
| 1 | 8 | 1 | 202 | 0.44 | 14 | 0.074 | 1.222 |
| 13 | 9 | −1 | 425 | 0.65 | 10 | 0.051 | 0.616 |
| 16 | 10 | 0 | 425 | 0.65 | 20 | 0.225 | 0.443 |
| 7 | 11 | 1 | 202 | 0.86 | 26 | 0.323 | 1.553 |
| 15 | 12 | 0 | 425 | 0.65 | 20 | 0.124 | 0.541 |
| 10 | 13 | −1 | 800 | 0.65 | 20 | 0.033 | 0.838 |
| 2 | 14 | 1 | 648 | 0.44 | 14 | 0.078 | 0.697 |
| 17 | 15 | 0 | 425 | 0.65 | 20 | 0.094 | 0.738 |
| 9 | 16 | −1 | 50 | 0.65 | 20 | 0.167 | 1.642 |
| 6 | 17 | 1 | 648 | 0.44 | 26 | 0.001 | 0.506 |
| 4 | 18 | 1 | 648 | 0.86 | 14 | 0.069 | 0.532 |
| 8 | 19 | 1 | 648 | 0.86 | 26 | 0.139 | 0.479 |
| 18 | 20 | 0 | 425 | 0.65 | 20 | 0.118 | 0.579 |
| Experimental Value (µA/mM) | Fit (µA/mM) | 95% PI (From Design) | |
|---|---|---|---|
| S Bi3+ | 1.804 | 2.684 | (1.759; 3.608) |
| S Al3+ | 0.524 | 0.574 | (0.095; 1.054) |
| Metal Ion | LOD (µM) | Upper limit of Linearity (µM) | Sensitivity (µA mM−1) |
|---|---|---|---|
| Bi3+ | 3.9 | 125 | 1.80 ± 0.12 |
| Al3+ | 16 | 500 | 0.52 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Benedetto, G.E.; Di Masi, S.; Pennetta, A.; Malitesta, C. Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection. Biosensors 2019, 9, 26. https://doi.org/10.3390/bios9010026
De Benedetto GE, Di Masi S, Pennetta A, Malitesta C. Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection. Biosensors. 2019; 9(1):26. https://doi.org/10.3390/bios9010026
Chicago/Turabian StyleDe Benedetto, Giuseppe Egidio, Sabrina Di Masi, Antonio Pennetta, and Cosimino Malitesta. 2019. "Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection" Biosensors 9, no. 1: 26. https://doi.org/10.3390/bios9010026
APA StyleDe Benedetto, G. E., Di Masi, S., Pennetta, A., & Malitesta, C. (2019). Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection. Biosensors, 9(1), 26. https://doi.org/10.3390/bios9010026