Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat
Abstract
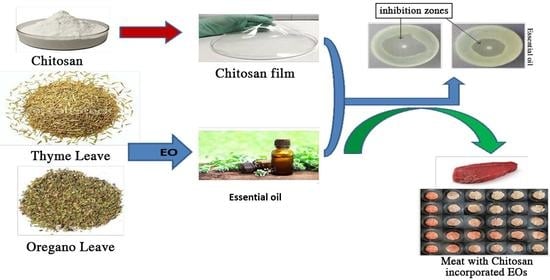
:1. Introduction
2. Results and Discussion
2.1. GC/MS-MS Fingerprint and Characterization of Essential Oil
2.2. Phenolics and Antioxidants Activity
2.3. Antibacterial Activity of CH Films Containing OO and TO Using Plate Overlay Assays (In Vitro)
2.4. Impact of CH Films Containing OO and TO on Background Microflora of Beef Meat (In Vivo)
2.4.1. Psychrophilic Bacteria
2.4.2. Pseudomonas Bacteria
2.4.3. Lactic Acid Bacteria (LAB)
2.5. Impact of CH Films Incorporating OO and TO on Foodborne Pathogens of Beef Meat (In Vivo)
2.6. Impact of CH Films Incorporating OO and TO on Meat Color during Storage Time
2.7. Sensory Evaluation
3. Materials and Methods
3.1. Raw Materials
3.2. Microorganisms
3.3. Essential Oil Extraction
3.4. GC/MS-MS Fingerprint
3.5. Chitosan Film Preparation
3.6. Plate Overlay Assays with Essential Oils
3.7. Challenge Studies
3.7.1. Trial I (Natural Microbiota)
3.7.2. Trial II (Pathogens Bacteria)
3.8. Microbiological Analyses
3.9. Phenolics and Antioxidants Activity
3.10. Color Measurement
3.11. Sensory Evaluation
3.12. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- CDC. Center for Disease Control. Surveillance for Foodborne Disease Outbreaks United States, 2015: Annual Report. Cdc, 1–24. 2017. Available online: https://www.cdc.gov/foodsafety/pdfs/2015FoodBorneOutbreaks_508.pdf (accessed on 12 February 2021).
- Bell, R.G. Meat packaging: Protection, preservation and presentation. Meat Sci. Appl. 2001, 463–490. [Google Scholar]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef]
- Lorenzo, J.; Domínguez, R.; Carballo, J. Control of lipid oxidation in muscle food by active packaging technology. In Natural Antioxidants: Applications in Foods of Animal Origin; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 343–382. [Google Scholar]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of fresh foal meat. Meat Sci. 2012, 91, 513–520. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- FDA. Essential oils, oleoresins (solvent-free), and natural extractives (including distillates). In Substances Generally Recognized as Safe; Foods and Drugs Administration, Department of Health and Human Services: Baltimore, MD, USA, 2017; Volume 21. [Google Scholar]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Agrimonti, C.; White, J.C.; Tonetti, S.; Marmiroli, N. Antimicrobial activity of cellulosic pads amended with emulsions of essential oils of oregano, thyme and cinnamon against microorganisms in minced beef meat. Int. J. Food Microbiol. 2019, 305, 108246. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef]
- Pedonese, F.; Fratini, F.; Pistelli, L.; Porta, F.M.; Di Ciccio, P.; Fischetti, R.; Turchi, B.; Nuvoloni, R. Antimicrobial activity of four essential oils against pigmenting Pseudomonas fluorescens and biofilmproducing Staphylococcus aureus of dairy origin. Ital. J. Food Saf. 2017, 6, 6939. [Google Scholar] [CrossRef]
- Yun, J.; Wu, C.; Li, X.; Fan, X. Improving the Microbial Food Safety of Fresh Fruits and Vegetables with Aqueous and Vaporous Essential Oils. In Natural and Bio-Based Antimicrobials for Food Applications; ACS Publications: Columbus, OH, USA, 2018; pp. 87–117. [Google Scholar]
- Pourhosseini, S.H.; Ahadi, H.; Aliahmadi, A.; Mirjalili, M.H. Chemical Composition and Antibacterial Activity of the Carvacrol-rich Essential Oils of Zataria multiflora Boiss.(Lamiaceae) from Southern Natural Habitats of Iran. J. Essent. Oil Bear. Plants 2020, 23, 779–787. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and evaluation of antioxidant packaging films made of polylactic acid containing thyme, rosemary, and oregano essential oils. J. Food Processing Preserv. 2019, 43, e14102. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Beuchat, L.R.; Kim, Y.; Ryu, J.-H. Synergistic antimicrobial activity of oregano and thyme thymol essential oils against Leuconostoc citreum in a laboratory medium and tomato juice. Food Microbiol. 2020, 90, 103489. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Bonilla, J.; de Sousa, R.L.M.; Moreno, A.M.; Baruselli, P.S. Chemical composition and antibacterial activity of essential oils against pathogens often related to cattle endometritis. J. Infect. Dev. Ctries 2020, 14, 177–183. [Google Scholar] [CrossRef]
- Xue, J.; Davidson, P.M.; Zhong, Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013, 61, 12720–12726. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Patterson, J.E.; McElmeel, L.; Wiederhold, N.P. In vitro activity of essential oils against Gram-positive and Gram-negative clinical isolates, including carbapenem-resistant Enterobacteriaceae. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2019; p. ofz502. [Google Scholar]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef]
- Adams, A.; Kruma, Z.; Verhé, R.; De Kimpe, N.; Kreicbergs, V. Volatile profiles of rapeseed oil flavored with basil, oregano, and thyme as a function of flavoring conditions. J. Am. Oil Chem. Soc. 2011, 88, 201–212. [Google Scholar] [CrossRef]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Di Santo, M.C.; Alaimo, A.; Rubio, A.P.D.; De Matteo, R.; Pérez, O.E. Biocompatibility analysis of high molecular weight chitosan obtained from Pleoticus muelleri shrimps. Evaluation in prokaryotic and eukaryotic cells. Biochem. Biophys. Rep. 2020, 24, 100842. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.; Ilyas, R. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-based edible coatings for the preservation of fishery products: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020, 162, 107961. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. Rsc Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef]
- Naseri, H.R.; Beigmohammadi, F.; Mohammadi, R.; Sadeghi, E. Production and characterization of edible film based on gelatin–chitosan containing Ferulago angulate essential oil and its application in the prolongation of the shelf life of turkey meat. J. Food Process. Preserv. 2020, 44, e14558. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Ayaseh, A.; Alirezalu, K.; Nemati, Z.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers 2021, 13, 716. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Radünz, M.; Camargo, T.M.; dos Santos Hackbart, H.C.; Alves, P.I.C.; Radünz, A.L.; Gandra, E.A.; da Rosa Zavareze, E. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Mutlutlugok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Kačániová, M.; Vukovič, N.; Hleba, L.; Bobková, A.; Pavelková, A.; Rovná, K.; Arpášová, H. Antimicrobial and antiradicals activity of Origanum vulgare L. and Thymus vulgaris essential oils. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 263–271. [Google Scholar]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.-P.; Zhang, X.; Pan, D.-D.; Sun, Y.-Y.; Cao, J.-X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of origanum vulgare l. Essential oil from the arid andean region of chile and its chemical characterization by gc-ms. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Jin, T.Z.; Chen, W.; He, Q.; Zou, Z.; Zhao, H.; Ye, X.; Guo, M. Preparation and characterization of gellan gum-chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocoll. 2021, 114, 106570. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Majdinasab, M.; Niakousari, M.; Shaghaghian, S.; Dehghani, H. Antimicrobial and antioxidant coating based on basil seed gum incorporated with Shirazi thyme and summer savory essential oils emulsions for shelf-life extension of refrigerated chicken fillets. Food Hydrocoll. 2020, 108, 106011. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Y.; Majura, J.J.; Qiu, Y.; Ding, J.; Hatab, S.; Miao, W.; Gao, Y. Combined Effect of the Essential Oil and Collagen Film on the Quality of Pacific Mackerel (Pneumatophorus japonicus) Fillet During Cold Storage. Foodborne Pathog. Dis. 2021, 18, 455–461. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Kulawik, P. The antimicrobial effect of grapefruit peel essential oil and its nanoemulsion on fish spoilage bacteria and food-borne pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Ayari, S.; Shankar, S.; Follett, P.; Hossain, F.; Lacroix, M. Potential synergistic antimicrobial efficiency of binary combinations of essential oils against Bacillus cereus and Paenibacillus amylolyticus-Part A. Microb. Pathog. 2020, 141, 104008. [Google Scholar] [CrossRef]
- Ghaderi, L.; Moghimi, R.; Aliahmadi, A.; McClements, D.; Rafati, H. Development of antimicrobial nanoemulsionionbinary combinations of essential oils against Bacillus cereus and Paenibacillus amylolytiensis essential oil. J. Appl. Microbiol. 2017, 123, 832–840. [Google Scholar] [CrossRef]
- Maggini, V.; Pesavento, G.; Maida, I.; Nostro, A.L.; Calonico, C.; Sassoli, C.; Perrin, E.; Fondi, M.; Mengoni, A.; Chiellini, C. Exploring the effect of the composition of three different Oregano essential oils on the growth of multidrug-resistant cystic fibrosis Pseudomonas aeruginosa strains. Nat. Prod. Commun. 2017, 12, 1934578X1701201234. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, L. Thymol as a critical component of Thymus vulgaris L. essential oil combats Pseudomonas aeruginosa by intercalating DNA and inactivating biofilm. LWT 2021, 136, 110354. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Fujino, N.; Sawaguchi, Y.; Sato, M.; Kuda, T.; Kimura, B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT 2021, 145, 111263. [Google Scholar] [CrossRef]
- de Souza, E.L. Insights into the current evidence on the effects of essential oils toward beneficial microorganisms in foods with a special emphasis to lactic acid bacteria–A review. Trends Food Sci. Technol. 2021, 114, 333–341. [Google Scholar] [CrossRef]
- Badia, V.; de Oliveira, M.S.R.; Polmann, G.; Milkievicz, T.; Galvão, A.C.; da Silva Robazza, W. Effect of the addition of antimicrobial oregano (Origanum vulgare) and rosemary (Rosmarinus officinalis) essential oils on lactic acid bacteria growth in refrigerated vacuum-packed Tuscan sausage. Braz. J. Microbiol. 2020, 51, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Yemiş, G.P.; Candoğan, K. Antibacterial activity of soy edible coatings incorporated with thyme and oregano essential oils on beef against pathogenic bacteria. Food Sci. Biotechnol. 2017, 26, 1113–1121. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Lu, X.; Mao, Y.; Luo, X.; Liu, G.; Zhu, L.; Zhang, Y. Effect of chitosan coating incorporated with oregano or cinnamon essential oil on the bacterial diversity and shelf life of roast duck in modified atmosphere packaging. Food Res. Int. 2021, 147, 110491. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for extracting essential oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–38. [Google Scholar]
- Li, Y.-q.; Kong, D.-x.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Siragusa, G.; Cutter, C.N.; Willett, J. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.; Fernández-López, J.; Abd ElRazik, K.; Omer, E.; Pérez-Alvarez, J.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Soni, A.; Gurunathan, K.; Mendiratta, S.K.; Talukder, S.; Jaiswal, R.K.; Sharma, H. Effect of essential oils incorporated edible film on quality and storage stability of chicken patties at refrigeration temperature (4 ± 1 C). J. Food Sci. Technol. 2018, 55, 3538–3546. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics A Biometrical Approach, 3rd ed.; McGraw Hill Book Company Inc.: New York, NY, USA, 1996; pp. 334–381. [Google Scholar]









| Sample | TP (mg GAE * L−1 Sample) | IC50 Inhibition (%) |
|---|---|---|
| Thyme essential oil | 201.52 ± 1.67 a | 58.44 ± 0.83 a |
| Oregano essential oil | 187.64 ± 1.65 b | 54.58 ± 0.62 b |
| Bacterial Strains | Antimicrobial Activity (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Oregano Oil | Thyme Oil | |||||||
| OO 0.5% | OO 1% | CH-OO 0.5% | CH-OO 1% | TO 0.5% | TO 1% | CH-TO 0.5% | CH-TO 1% | |
| E. coli O157:H7 | 15 ± 1.17 cE | 21 ± 1.26 cB | 18 ± 1.11 bC | 24 ± 1.03 bA | 16 ± 1.15 bD | 20 ± 1.35 bB | 18 ± 1.03 bC | 23 ± 1.15 bA |
| S. aureus | 30 ± 1.45 aG | 45 ± 1.33 aD | 32 ± 1.32 aF | 47 ± 1.5 aC | 41 ± 1.6 aE | 50 ± 3.65 aB | 44 ± 1.43 aD | 52 ± 1.85 aA |
| S. Typhimurium | 17 ± 1.11 bE | 23 ± 1.33 bB | 19 ± 1 bD | 25 ± 1.16 bA | 16 ± 1.24 bE | 21 ± 1.25 bC | 19 ± 1.37 bD | 24 ± 1.22 bA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaba, A.B.M.; Hassan, M.A.; Abd EL-Tawab, A.A.; Abdelmonem, M.A.; Morsy, M.K. Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat. Antibiotics 2022, 11, 583. https://doi.org/10.3390/antibiotics11050583
Gaba ABM, Hassan MA, Abd EL-Tawab AA, Abdelmonem MA, Morsy MK. Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat. Antibiotics. 2022; 11(5):583. https://doi.org/10.3390/antibiotics11050583
Chicago/Turabian StyleGaba, Abdul Basit M., Mohamed A. Hassan, Ashraf A. Abd EL-Tawab, Mohamed A. Abdelmonem, and Mohamed K. Morsy. 2022. "Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat" Antibiotics 11, no. 5: 583. https://doi.org/10.3390/antibiotics11050583
APA StyleGaba, A. B. M., Hassan, M. A., Abd EL-Tawab, A. A., Abdelmonem, M. A., & Morsy, M. K. (2022). Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat. Antibiotics, 11(5), 583. https://doi.org/10.3390/antibiotics11050583