Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids
Abstract
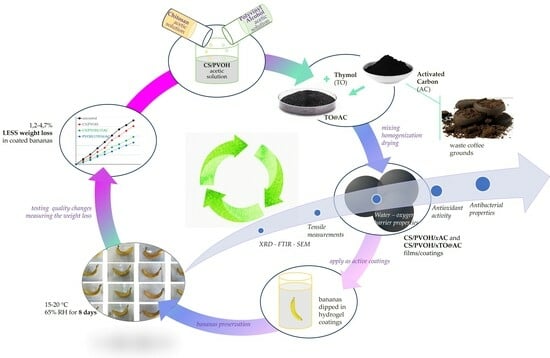
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TO@AC Nanohybrid
2.3. CS/PVOH/xAC and CS/PVOH/xTO@AC Films Preparation
2.4. XRD Analysis of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
2.5. FTIR Spectroscopy of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
2.6. Tensile Measurements of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
2.7. Water Vapor Transmission Rate Measurements and Water Diffusion Coefficient Calculation
2.8. Oxygen Transmission Rate Measurements and Oxygen Permeability Calculation
2.9. Total Antioxidant Activity of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
2.10. Antibacterial Activity Tests of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
2.11. Application in Fresh Bananas Preservation
2.12. Statistical Analysis
3. Results
3.1. XRD Analysis of CS/PVOH/xAC, CS/PVOH/xTO@AC Films
3.2. FTIR Spectroscopy of CS/PVOH/xAC, CS/PVOH/xTO@AC Films
3.3. Tensile Properties CS/PVOH/xAC, CS/PVOH/xTO@AC Films
3.4. Water-Oxygen Barrier Properties of CS/PVOH/xAC, CS/PVOH/xTO@AC Films
3.5. Total Antioxidant Activity of CS/PVOH/xAC and CS/PVOH/xTO@AC Films
3.6. Antibacterial Properties of CS/PVOH/xAC and CS/PVOH/xTO@AC Films Agar Diffusion Test
3.7. Visual Evaluation of the Obtained Active Coatings against Enzymatic Browning of Fresh Bananas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Viaggi, D. Agricultural Waste Management and Valorisation in the Context of the Circular Bioeconomy: Exploring the Potential of Biomass Value Webs. Curr. Opin. Environ. Sci. Health 2022, 27, 100356. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef]
- Holden, N.M.; White, E.P.; Lange, M.C.; Oldfield, T.L. Review of the Sustainability of Food Systems and Transition Using the Internet of Food. npj Sci. Food 2018, 2, 18. [Google Scholar] [CrossRef]
- Nemat, B.; Razzaghi, M.; Bolton, K.; Rousta, K. The Role of Food Packaging Design in Consumer Recycling Behavior—A Literature Review. Sustainability 2019, 11, 4350. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A Roadmap towards Green Packaging: The Current Status and Future Outlook for Polyesters in the Packaging Industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Kumar Nadda, A.; Saad Bala Husain, M.; Gupta, A. Biopolymers from Waste Biomass and Its Applications in the Cosmetic Industry: A Review. Mater. Today Proc. 2022, 68, 873–879. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Package Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Aider, M. Chitosan Application for Active Bio-Based Films Production and Potential in the Food Industry: Review. LWT—Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Chowdhury, S.A.; Dabnichki, P.; Ahmed, N.; Arefin, A.M.E. Sustainable Food Waste Management Model for Bangladesh. Sustain. Prod. Consum. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Huang, I.Y.; Manning, L.; James, K.L.; Grigoriadis, V.; Millington, A.; Wood, V.; Ward, S. Food Waste Management: A Review of Retailers’ Business Practices and Their Implications for Sustainable Value. J. Clean. Prod. 2021, 285, 125484. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation and Industrial Uses: A Review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Thi, N.B.D.; Kumar, G.; Lin, C.-Y. An Overview of Food Waste Management in Developing Countries: Current Status and Future Perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food Waste and the Food-Energy-Water Nexus: A Review of Food Waste Management Alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A Critical Review on Intelligent and Active Packaging in the Food Industry: Research and Development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of Natural Zeolites on Agriculture and Food Production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Activated Carbon for Food Packaging Application: Review. Walailak J. Sci. Technol. 2018, 15, 255–271. [Google Scholar] [CrossRef]
- Quevedo, R.; Díaz, O.; Ronceros, B.; Pedreschi, F.; Aguilera, J.M. Description of the Kinetic Enzymatic Browning in Banana (Musa Cavendish) Slices Using Non-Uniform Color Information from Digital Images. Food Res. Int. 2009, 42, 1309–1314. [Google Scholar] [CrossRef]
- Kaewjumpol, G.; Srisamlee, S.; Beckles, D.M.; Luengwilai, K. Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction. Horticulturae 2021, 7, 373. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Li, J.; Yang, Q. A Novel Edible Packaging Film Based on Chitosan Incorporated with Persimmon Peel Extract for the Postharvest Preservation of Banana. Food Qual. Saf. 2022, 6, fyac028. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous Activated Carbon Derived via Pyrolysis Process of Spent Coffee: Structural Characterization. Investigation of Its Use for Hexavalent Chromium Removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef]
- Salmas, C.Ε.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Poly-Vinyl-Alcohol Based Hydrogels Applied as Active Pads for Strawberries Preservation. Gels 2023, 9, 570. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- John, J.; Noorjan, N.; Gurumurthy, S.C.; Ramaprasad, A.T. Chitosan-Polyvinyl Alcohol Blend as Beta-Ray Attenuator. Mater. Today Proc. 2022, 66, 2109–2114. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Chapter Two—Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Carbohydrates: Fundamentals and Applications, Part B; Academic Press: Cambridge, MA, USA, 2014; Volume 73, pp. 15–31. [Google Scholar]
- Sallam, M.F.; Ahmed, H.M.S.; Diab, K.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Sharaf, H.A.; Abdel-Wahhab, M.A. Improvement of the Antioxidant Activity of Thyme Essential Oil against Biosynthesized Titanium Dioxide Nanoparticles-Induced Oxidative Stress, DNA Damage, and Disturbances in Gene Expression in Vivo. J. Trace Elem. Med. Biol. 2022, 73, 127024. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Ugwuja, C.G.; Omorogie, M.O.; Adewuyi, A.; Oladoja, N.A. Clays for Efficient Disinfection of Bacteria in Water. Appl. Clay Sci. 2018, 151, 211–223. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T. Antibacterial and Adsorption Characteristics of Activated Carbon Functionalized with Quaternary Ammonium Moieties. Ind. Eng. Chem. Res. 2007, 46, 439–445. Available online: https://pubs.acs.org/doi/10.1021/ie0608096 (accessed on 10 August 2023). [CrossRef]
- Stewart, M.H.; Wolfe, R.L.; Means, E.G. Assessment of the Bacteriological Activity Associated with Granular Activated Carbon Treatment of Drinking Water. Appl. Environ. Microbiol. 2023, 56, 3822–3829. Available online: https://journals.asm.org/doi/10.1128/aem.56.12.3822-3829.1990 (accessed on 17 July 2023). [CrossRef]
- Burchacka, E.; Pstrowska, K.; Beran, E.; Fałtynowicz, H.; Chojnacka, K.; Kułażyński, M. Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens 2021, 10, 1066. Available online: https://www.mdpi.com/2076-0817/10/8/1066 (accessed on 17 July 2023). [CrossRef] [PubMed]
- Azmi, N.N.; Mahyudin, N.A.; Wan Omar, W.H.; Mahmud Ab Rashid, N.-K.; Ishak, C.F.; Abdullah, A.H.; Sharples, G.J. Antibacterial Activity of Clay Soils against Food-Borne Salmonella Typhimurium and Staphylococcus Aureus. Molecules 2022, 27, 170. [Google Scholar] [CrossRef]








| Sample Code Name | E | σ uts | ε% |
|---|---|---|---|
| CS/PVOH | 2249.3(100.3) | 71.2(1.8) | 11.8(0.9) |
| CS/PVOH/5AC | 2511.0(43.0) | 79.0(5.7) | 4.1(0.7) |
| CS/PVOH/10AC | 2881.3(63.2) | 86.1(6.1) | 3.4(0.1) |
| CS/PVOH/15AC | 2789.0(64.4) | 82.1(4.5) | 5.4(0.9) |
| CS/PVOH/5TO@AC | 2692.0(54.2) | 81.8(7.4) | 5.2(1.5) |
| CS/PVOH10TO@AC | 3041.3(79.2) | 104.3(8.0) | 6.1(0.9) |
| CS/PVOH/15TO@AC | 2938.5(60.5) | 88.0(6.1) | 5.9(0.8) |
| Sample Code Name | Water Vapor Transmission Rate Film Thickness (mm) | Water Vapor Transmission Rate (10−6 g/cm2.s) | Diffusion Coefficient (10−4 cm2/s) | Oxygen Transmission Rate Film Thickness (mm) | Oxygen Transmission Rate (mL/m2.day) | PeO2 (10−7 cm2/s) |
|---|---|---|---|---|---|---|
| CS/PVOH | 0.14 ± 0.01 | 1.339 ± 0.024 | 4.36 ± 0.11 | 0.15 ± 0.01 | 38.20 ± 1.91 | 5.73 ± 0.29 |
| CS/PVOH/5AC | 0.15 ± 0.01 | 1.240 ± 0.025 | 4.32 ± 0.11 | 0.15 ± 0.01 | 19.10 ± 0.96 | 2.87 ± 0.14 |
| CS/PVOH/10AC | 0.17 ± 0.01 | 0.896 ± 0.021 | 3.53 ± 0.10 | 0.09 ± 0.01 | 27.50 ± 1.38 | 2.48 ± 0.12 |
| CS/PVOH/15AC | 0.16 ± 0.01 | 0.924 ± 0.014 | 3.43 ± 0.07 | 0.16 ± 0.01 | 10.10 ± 0.51 | 1.62 ± 0.08 |
| CS/PVOH/5TO@AC | 0.12 ± 0.01 | 0.902 ± 0.018 | 3.18 ± 0.07 | 0.14 ± 0.01 | 18.50 ± 0.93 | 2.59 ± 0.13 |
| CS/PVOH/10TO@AC | 0.13 ± 0.01 | 0.735 ± 0.017 | 2.99 ± 0.09 | 0.10 ± 0.01 | 17.50 ± 0.88 | 1.75 ± 0.09 |
| CS/PVOH/15TO@AC | 0.14 ± 0.01 | 0.802 ± 0.012 | 2.17 ± 0.09 | 0.15 ± 0.01 | 10.30 ± 0.50 | 1.49 ± 0.07 |
| Film Material | E. coli | S. aureus | S. enterica | L. monocytogenes |
|---|---|---|---|---|
| Inhibition 1 (Diameter of Clear Zone) | Inhibition 1 (Diameter of Clear Zone) | Inhibition 1 (Diameter of Clear Zone) | Inhibition 1 (Diameter of Clear Zone) | |
| CS | 3.07 ± 0.22 a | 3.56 ± 0.43 a | 3.40 ± 0.32 a | 2.03 ± 0.26 a |
| CS/PVOH | 3.63 ± 0.35 ab | 3.72 ± 0.25 ab | 3.49 ± 0.11 ab | 3.78 ± 0.52 ab |
| CS/PVOH/5AC | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 0 ± 0 ac |
| CS/PVOH/10AC | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 0 ± 0 ac |
| CS/PVOH/15AC | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c | 0 ± 0 ac |
| CS/PVOH/5TO@AC | 3.08 ± 0.26 abd | 5.43 ± 0.49 abd | 4.13 ± 0.68 abd | 2.66 ± 0.36 a |
| CS/PVOH/10TO@AC | 3.10 ± 0.29 abd | 3.63 ± 0.46 abd | 3.00 ± 0.16 abd | 3.44 ± 0.30 ad |
| CS/PVOH/15TO@AC | 3.84 ± 0.60 abd | 4.53 ± 0.36 abd | 6.07 ± 0.18 e | 3.86 ± 0.91 ad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmas, C.E.; Leontiou, A.; Kollia, E.; Zaharioudakis, K.; Kopsacheili, A.; Avdylaj, L.; Georgopoulos, S.; Karabagias, V.K.; Karydis-Messinis, A.; Kehayias, G.; et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings 2023, 13, 1503. https://doi.org/10.3390/coatings13091503
Salmas CE, Leontiou A, Kollia E, Zaharioudakis K, Kopsacheili A, Avdylaj L, Georgopoulos S, Karabagias VK, Karydis-Messinis A, Kehayias G, et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings. 2023; 13(9):1503. https://doi.org/10.3390/coatings13091503
Chicago/Turabian StyleSalmas, Constantinos E., Areti Leontiou, Eleni Kollia, Konstantinos Zaharioudakis, Anna Kopsacheili, Learda Avdylaj, Stavros Georgopoulos, Vassilios K. Karabagias, Andreas Karydis-Messinis, George Kehayias, and et al. 2023. "Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids" Coatings 13, no. 9: 1503. https://doi.org/10.3390/coatings13091503
APA StyleSalmas, C. E., Leontiou, A., Kollia, E., Zaharioudakis, K., Kopsacheili, A., Avdylaj, L., Georgopoulos, S., Karabagias, V. K., Karydis-Messinis, A., Kehayias, G., Proestos, C., & Giannakas, A. E. (2023). Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings, 13(9), 1503. https://doi.org/10.3390/coatings13091503