Establishment of Wild-Derived Strains of Japanese Quail (Coturnix japonica) in Field and Laboratory Experiments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
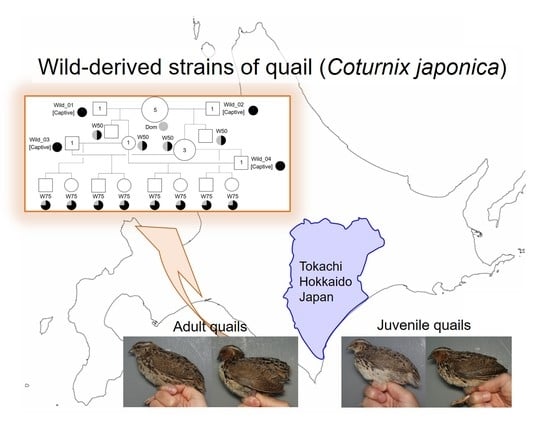
2.1. Field Study
2.2. Laboratory Experiment
2.3. Statistical Analysis
3. Results
3.1. Field Study
3.2. Morphological Analysis of Captive Wild Quails
3.3. Establishment of Wild-derived Strains of Japanese Quail
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minvielle, F. The future of Japanese quail for research and production. Worlds Poult. Sci. J. 2004, 60, 500–507. [Google Scholar] [CrossRef]
- Mizutani, M. The Japanese quail. In The Relationships between Indigenous Animals and Humans in APEC Region; Chinese Society of Animal Science: Taipei, Taiwan, 2003; pp. 143–163. [Google Scholar]
- Tsudzuki, M. Mutations of Japanese quail (Coturnix japonica) and recent advances of molecular genetics for this species. J. Poult. Sci. 2008, 45, 159–179. [Google Scholar] [CrossRef] [Green Version]
- Kawahara-Miki, R.; Sano, S.; Nunome, M.; Shimmura, T.; Kuwayama, T.; Takahashi, S.; Kawashima, T.; Matsuda, Y.; Yoshimura, T.; Kono, T. Next-generation sequencing reveals genomic features in the Japanese quail. Genomics 2013, 101, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, K.M.; Hindle, M.M.; Boitard, S.; Burt, D.W.; Danner, A.F.; Eory, L.; Forrest, H.L.; Gourichon, D.; Gros, J.; Hillier, L.W.; et al. The quail genome: Insights into social behaviour, seasonal biology and infectious disease response. BMC Biol. 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhang, Y.; Hou, Z.; Fan, G.; Pi, J.; Sun, S.; Chen, J.; Liu, H.; Du, X.; Shen, J.; et al. Population genomic data reveal genes related to important traits of quail. Gigascience 2018, 7, giy049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minvielle, F.; Kayang, B.B.; Inoue-Murayama, M.; Miwa, M.; Vignal, A.; Gourichon, D.; Neau, A.; Monvoisin, J.L.; Ito, S.I. Search for QTL affecting the shape of the egg laying curve of the Japanese quail. BMC Genet. 2006, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Vollmar, S.; Haas, V.; Schmid, M.; Preuß, S.; Joshi, R.; Rodehutscord, M.; Bennewitz, J. Mapping genes for phosphorus utilization and correlated traits using a 4k SNP linkage map in Japanese quail (Coturnix japonica). Anim. Genet. 2021, 52, 90–98. [Google Scholar] [CrossRef]
- Haqani, M.I.; Nomura, S.; Nakano, M.; Goto, T.; Nagano, A.J.; Takenouchi, A.; Nakamura, Y.; Ishikawa, A.; Tsudzuki, M. Mapping of quantitative trait loci controlling egg-quality and -production traits in Japanese quail (Coturnix japonica) using restriction-site associated DNA sequencing. Genes 2021, 12, 735. [Google Scholar] [CrossRef]
- Haqani, M.I.; Nomura, S.; Nakano, M.; Goto, T.; Nagano, A.J.; Takenouchi, A.; Nakamura, Y.; Ishikawa, A.; Tsudzuki, M. Quantitative trait loci for growth-related traits in Japanese quail (Coturnix japonica) using restriction-site associated DNA sequencing. Mol. Genet. Genom. 2021, 296, 1147–1159. [Google Scholar] [CrossRef]
- Goto, T.; Tsudzuki, M. Genetic mapping of quantitative trait loci for egg production and egg quality traits in chickens: A review. J. Poult. Sci. 2017, 54, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, T. Body traits and behavior in wild Japanese quail Coturnix coturnix japonica. Jpn. J. Ornithol. 1978, 27, 105–112. [Google Scholar] [CrossRef]
- Lukanov, H.; Pavlova, I. Domestication changes in Japanese quail (Coturnix japonica): A review. Worlds Poult. Sci. J. 2020, 76, 787–801. [Google Scholar] [CrossRef]
- Okuyama, M. Current status of Japanese quail Coturnix japonica as a game bird. J. Yamashina Inst. Ornithol. 2004, 35, 189–202. [Google Scholar] [CrossRef]
- Fujimaki, Y. Status and distribution of the Japanese quail Coturnix japonica in central and southeastern Hokkaido, Japan. Jpn. J. Ornithol. 2010, 59, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Ministry of the Environment. The 3rd Version of the Japanese Red Lists; Japan Wildlife Research Center: Tokyo, Japan, 2006. [Google Scholar]
- Kimura, M.; Fujii, S. Genetic variability within and between wild and domestic Japanese quail population. Jpn. Poult. Sci. 1989, 26, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.E.; Zdraljevic, S.; Roberts, J.P.; Andersen, E.C. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 2017, 45, D650–D657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lollar, M.J.; Biewer-Heisler, T.J.; Danen, C.E.; Pool, J.E. Hybrid breakdown in male reproduction between recently-diverged Drosophila melanogaster populations has a complex and variable genetic architecture. Evolution 2023, 77, 1550–1563. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Shiroishi, T.; Koide, T. Genetic mapping of escalated aggression in wild-derived mouse strain MSM/Ms: Association with serotonin-related genes. Front. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.S.; van Wijk, M.H.; McGrath, P.T.; Andersen, E.C.; Sterken, M.G. From QTL to gene: C. elegans facilitates discoveries of the genetic mechanisms underlying natural variation. Trends Genet. 2021, 37, 933–947. [Google Scholar] [CrossRef]
- Lack, J.B.; Lange, J.D.; Tang, A.D.; Corbett-Detig, R.B.; Pool, J.E. A thousand fly genomes: An expanded Drosophila genome nexus. Mol. Biol. Evol. 2016, 33, 3308–3313. [Google Scholar] [CrossRef] [Green Version]
- Churchill, G.A.; Gatti, D.M.; Munger, S.C.; Svenson, K.L. The diversity outbred mouse population. Mamm. Genome. 2012, 23, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Goto, T.; Nishino, J.; Nakaoka, H.; Tanave, A.; Takano-Shimizu, T.; Mott, R.F.; Koide, T. Selective breeding and selection mapping using a novel wild-derived heterogeneous stock of mice revealed two closely-linked loci for tameness. Sci. Rep. 2017, 7, 4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deregnaucourt, S.; Guyomarc’h, J.-C. Mating call discrimination in female European (Coturnix c. coturnix) and Japanese quail (Coturnix c. japonica). Ethology 2003, 109, 107–119. [Google Scholar] [CrossRef]
- Desmedt, L.; George, I.; Mohamed Benkada, A.; Hervé, M.; Aubin, T.; Derégnaucourt, S.; Lumineau, S. Maternal presence influences vocal development in the Japanese quail (Coturnix c. japonica). Ethology 2020, 126, 553–562. [Google Scholar] [CrossRef]
- Cramp, S.; Snow, D.W.; Perrins, C.M. (Eds.) The Complete Birds of the Western Paleartic; CD-ROM v.1.0; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Bird Migration Research Center. Bird Banding Manual (Revised ), 11th ed.; Yamashina Institute for Ornithology: Abiko, Japan, 2009; pp. 1–96. Available online: https://www.biodic.go.jp/banding/pdf/banding_manual.pdf (accessed on 1 June 2023).
- Konno, S.; Murakami, H.; Matsuo, T. Knowledge about Rufous-tailed Robin Luscinia sibilans, Dusky Warbler Phylloscopus fuscatus, and Radde’s Warbler Phylloscopus schwarzi obtained during Japan-Russia bird banding expedition on Sakhalin island. Bull Jpn. Bird Band. Assoc. 2013, 25, 65–76. [Google Scholar] [CrossRef]
- Svensson, L. Identification Guide to European Passerines, Revised and Enlarged Edition; Published by the Author: Stockholm, Sweden, 1992; pp. 1–368. [Google Scholar]
- Lyon, D.L. Comparative growth and plumage development in Coturnix and Bobwhite. Wilson Bull. 1962, 74, 5–27. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 June 2023).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio: PBC, Boston, MA, 2022; Available online: http://www.rstudio.com/ (accessed on 1 June 2023).
- Ainsworth, S.J.; Stanley, R.L.; Evans, D.J. Developmental stages of the Japanese quail. J. Anat. 2010, 216, 3–15. [Google Scholar] [CrossRef]
- Nunome, M.; Nakano, M.; Tadano, R.; Kawahara-Miki, R.; Kono, T.; Takahashi, S.; Kawashima, T.; Fujiwara, A.; Nirasawa, K.; Mizutani, M.; et al. Genetic divergence in domestic Japanese quail inferred from mitochondrial DNA D-loop and microsatellite markers. PLoS ONE 2017, 12, e0169978. [Google Scholar] [CrossRef] [Green Version]
- Nakane, Y.; Yoshimura, T. Photoperiodic regulation of reproduction in vertebrates. Annu. Rev. Anim. Biosci. 2019, 7, 173–194. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T. Discovery of gonadotropin-inhibitory hormone (GnIH), progress in GnIH research on reproductive physiology and behavior and perspective of GnIH research on neuroendocrine regulation of reproduction. Mol. Cell Endocrinol. 2020, 514, 110914. [Google Scholar] [CrossRef] [PubMed]





| Year | Number of Birds Captured | |||||
|---|---|---|---|---|---|---|
| Adult | Juvenile | |||||
| Female | Male | Total | Female | Male | Total | |
| 2019 | 0 | 2 | 2 | 0 | 1 | 1 |
| 2020 | 1 | 5 | 6 | 2 | 5 | 7 |
| 2021 | 0 | 8 | 8 | 2 | 0 | 2 |
| 2022 | 0 | 4 | 4 | 1 | 0 | 1 |
| Total | 1 | 19 | 20 | 5 | 6 | 11 |
| Trait | Age (Adult) | Age (Juvenile) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| n = 1 | n = 16–19 | n = 4–5 | n = 6 | |
| Body weight (g) | 118.7 | 98.0 ± 5.6 | 84.3 ± 14.0 | 90.9 ± 7.0 |
| Tarsus length (mm) | 27.0 | 26.7 ± 1.0 | 27.0 ± 0.5 | 27.4 ± 1.1 |
| Total head length (mm) | 36.3 | 35.7 ± 0.6 | 35.0 ± 0.9 | 35.9 ± 0.7 |
| Entire culmen (mm) | 16.5 | 15.9 ± 0.7 | 15.4 ± 0.6 | 15.4 ± 1.0 |
| Exposed culmen with cere (mm) | 13.7 | 13.5 ± 0.5 | 12.8 ± 1.0 | 13.2 ± 0.9 |
| Feather Generation (2nd+) | Feather Generation (1st) | |||
|---|---|---|---|---|
| Trait | Female | Male | Female | Male |
| n = 1 | n = 5 | n = 3 | n = 20 | |
| Natural wing length (mm) | 92.0 | 95.3 ± 2.3 | 95.0 ± 1.9 | 93.8 ± 1.7 |
| Maximum wing length (mm) | 103.5 | 104.3 ± 1.5 | 103.8 ± 1.6 | 102.8 ± 2.8 |
| Trait | Feather Generation (2nd+) | Feather Generation (1st) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| n = 2 | n = 18 | n = 3 | n = 4 | |
| Tail length (mm) | 38.0 ± 0.0 | 37.7 ± 2.5 | 36.5 ± 1.0 | 30.2 ± 2.7 |
| Trait 1 | Mean ± SE | Range | Mean ± SE | Range |
|---|---|---|---|---|
| 1914 population 2 | ||||
| Female (n = 6) | Male (n = 18) | |||
| (1) Body weight (g) | - | - | ||
| (2) Internal shank length (mm) | 26.6 ± 0.08 | 24.1–30.0 | 26.9 ± 0.03 | 25.4–30.0 |
| (3) Bill length (mm) | 13.1 ± 0.01 | 13.0–13.5 | 13.0 ± 0.01 | 12.7–13.5 |
| (4) Wing length (mm) | 98.3 ± 0.04 | 97.0–99.6 | 97.1 ± 0.04 | 94.2–99.6 |
| (5) Tail length (mm) | 38.6 ± 0.06 | 36.7–40.6 | 38.2 ± 0.05 | 35.3–41.9 |
| 1970 population 3 | ||||
| Female (n = 22) | Male (n = 17) | |||
| (1) Body weight (g) | 99.7 ± 1.51 | 90.0–115.0 | 96.4 ± 2.12 | 86.0–114.0 |
| (2) Internal shank length (mm) | 26.6 ± 0.30 | 23.75–28.90 | 25.4 ± 0.32 | 23.40–27.95 |
| (3) Bill length (mm) | 12.7 ± 0.11 | 11.25–13.55 | 12.6 ± 0.09 | 12.00–13.35 |
| (4) Wing length (mm) | 103.0 ± 0.44 | 99.80–107.60 | 102.1 ± 0.56 | 97.00–107.00 |
| (5) Tail length (mm) | 39.7 ± 0.69 | 33.00–42.85 | 40.3 ± 0.47 | 34.95–43.40 |
| 2019–2022 population 4 | ||||
| Female (n = 1) | Male (n = 19) | |||
| (1) Body weight (g) | 118.7 | 98.0 ± 1.3 | 85.9–106.8 | |
| (2) Tarsus length (mm) | 27.0 | 26.7 ± 0.2 | 24.5–29.1 | |
| (3) Exposed culmen with cere (mm) | 13.7 | 13.5 ± 0.1 | 12.5–14.6 | |
| Female (n = 1) 5 | Male (n = 5) 5 | |||
| (4) Natural wing length (mm) | 92.0 | 95.3 ± 2.3 | 91.5–97.3 | |
| Female (n = 2) 6 | Male (n = 18) 6 | |||
| (5) Tail length (mm) | 38.0 ± 0.0 | 38.0–38.0 | 37.7 ± 2.5 | 31.9–42.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Konno, S.; Konno, M. Establishment of Wild-Derived Strains of Japanese Quail (Coturnix japonica) in Field and Laboratory Experiments. Biology 2023, 12, 1080. https://doi.org/10.3390/biology12081080
Goto T, Konno S, Konno M. Establishment of Wild-Derived Strains of Japanese Quail (Coturnix japonica) in Field and Laboratory Experiments. Biology. 2023; 12(8):1080. https://doi.org/10.3390/biology12081080
Chicago/Turabian StyleGoto, Tatsuhiko, Satoshi Konno, and Miwa Konno. 2023. "Establishment of Wild-Derived Strains of Japanese Quail (Coturnix japonica) in Field and Laboratory Experiments" Biology 12, no. 8: 1080. https://doi.org/10.3390/biology12081080
APA StyleGoto, T., Konno, S., & Konno, M. (2023). Establishment of Wild-Derived Strains of Japanese Quail (Coturnix japonica) in Field and Laboratory Experiments. Biology, 12(8), 1080. https://doi.org/10.3390/biology12081080