Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin
Abstract
:1. Introduction
2. Biosynthesis of Melatonin
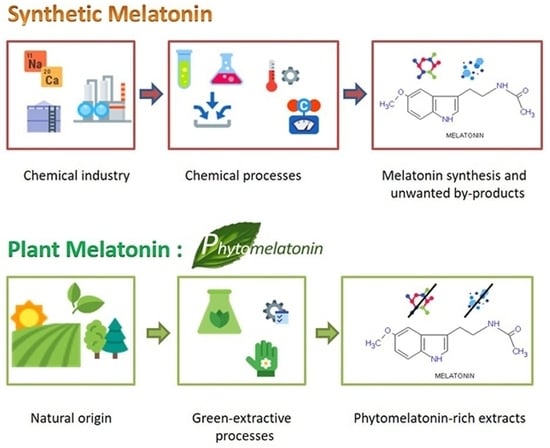
3. Biological Melatonin versus Synthetic Melatonin
4. Strategies to Obtain Biological Melatonin
4.1. Melatonin from Microorganisms
| Product | Biological Origin | Natural Level | Concentrated at | Trademark | Ref. |
|---|---|---|---|---|---|
| 1 | S. cerevisiae (GMO) | 85 µg·L−1 | 14.5 mg·L−1 | - Novo Nordisk Co. | [112] |
| 2 | E. coli (GMO) | ~ng·L−1 | 1–2 g·L−1 | - Novo Nordisk Co. | [97] |
| 3 | Lactobacillus and others | ng-µg·L−1 | 8 mg in liquid (0.015%) | Melatonin Qultured * Quantum Nutrition Labs | [114] |
| 4 | Chlorella Rice Alfalfa | 2–15 ng·g DW−1 1–5 ng·g DW−1 16 ng·g DW−1 | 0.3, 3 mg·pill (1.4%) | Herbatonin * Natural Health Int. Co. | [118] |
| 5 | Tart cherries | 14 ng·g FW−1 | 14 µg·pill (0.003%) | Sleep Support * Tru2U Co. | [121] |
| 6 | Rice seed | 7–216 ng·g DW−1 | - | - | [122,123,124] |
| 7 | Mustard seed | 194–660 ng·g DW−1 | - | - | [125,126] |
| 8 | St. John’s Wort | See Table 2 | 0.3, 1.9 mg·pill (1%) | Mélatonine Végétale * Dynveo Co. | - |
| 9 | Valerian root | 1.5–2 µg·g DW−1 | - | - UMU | [127] |
| 10 | MAPs | 1–20 µg·g DW−1 | - | Bioriex UMU | [128,129] |
4.2. Melatonin from Plants
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordor Intelligence. Melatonin Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/melatonin-market (accessed on 30 October 2022).
- Tan, D.-X.; Reiter, R.J. Mechanisms and Clinical Evidence to Support Melatonin’s Use in Severe COVID-19 Patients to Lower Mortality. Life Sci. 2022, 294, 120368. [Google Scholar] [CrossRef] [PubMed]
- Market Analysis Report Melatonin Market Size, Share & Trends Analysis Report By Application, Regional Outlook, Competitive Strategies, and Segment Forecasts, 2019 to 2025. Available online: https://www.grandviewresearch.com/industry-analysis/melatonin-market (accessed on 30 October 2022).
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Krause, D.; Dubocovich, M. Regulatory Sites in the Melatonin System of Mammals. Trends Neurosci. 1990, 13, 464–470. [Google Scholar] [CrossRef]
- Luo, C.; Yang, Q.; Liu, Y.; Zhou, S.; Jiang, J.; Reiter, R.J.; Bhattacharya, P.; Cui, Y.; Yang, H.; Ma, H.; et al. The Multiple Protective Roles and Molecular Mechanisms of Melatonin and Its Precursor N-Acetylserotonin in Targeting Brain Injury and Liver Damage and in Maintaining Bone Health. Free Radic. Biol. Med. 2019, 130, 215–233. [Google Scholar] [CrossRef]
- Fujii, R. The Regulation of Motile Activity in Fish Chromatophores. Pigment. Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef]
- López-Olmeda, J.; Madrid, J.; Sánchez-Vázquez, F. Melatonin Effects on Food Intake and Activity Rhythms in Two Fish Species with Different Activity Patterns: Diurnal (Goldfish) and Nocturnal (Tench). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 180–187. [Google Scholar] [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Human Diseases. Int. J. Mol. Sci. 2022, 23, 7438. [Google Scholar] [CrossRef]
- Rozov, S.V. Features of Melatonin Catabolism in Chicks. Neurochem. J. 2008, 2, 188–192. [Google Scholar] [CrossRef]
- de Pontes, M.P.; de Souza Khatlab, A.; Del Vesco, A.P.; Granzoto, G.H.; Soares, M.A.M.; de Sousa, F.C.B.; de Souza, M.L.R.; Gasparino, E. The Effect of Light Regime and Time of Slaughter in Broiler on Broiler Performance, Liver Antioxidant Status, and Expression of Genes Related to Peptide Absorption in the Jejunum and Melatonin Synthesis in the Brain. J. Anim. Physiol. Anim. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Vivien-Roels, B.; Pávet, P. Melatonin: Presence and Formation in Invertebrates. Experientia 1993, 49, 642–647. [Google Scholar] [CrossRef]
- Reiter, R.J.; Poeggeler, B.; Tan, D.X.; Chen, L.; Manchester, L.; Guerrero, J. Antioxidant Capacity of Melatonin. A Novel Action Not Requiring a Receptor. Neuroendocrinol. Lett. 1993, 15, 103–116. [Google Scholar]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A Potent, Endogenous Hydroxyl Radical Scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J. Interactions of the Pineal Hormone Melatonin with Oxygen-Centered Free-Radicals. A Brief Review. Braz. J. Med. Biol. Res. 1993, 26, 1141–1155. [Google Scholar]
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, M.A.; Suárez, S.; Puente-Moncada, N.; Anítua, J.M.; et al. Mechanisms Involved in the Pro-Apoptotic Effect of Melatonin in Cancer Cells. Int. J. Mol. Sci. 2013, 14, 6597–6613. [Google Scholar] [CrossRef]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer Metastasis: Mechanisms of Inhibition by Melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef]
- Loh, D.; Reiter, R. Melatonin: Regulation of Prion Protein Phase Separation in Cancer Multidrug Resistance. Molecules 2022, 27, 705. [Google Scholar] [CrossRef]
- Maqbool, S.; Ihtesham, A.; Langove, M.N.; Jamal, S.; Jamal, T.; Safian, H.A. Neuro-Dermatological Association between Psoriasis and Depression: An Immune-Mediated Inflammatory Process Validating Skin-Brain Axis Theory. AIMS Neurosci. 2021, 8, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, R.; Wang, Z.; Chen, Z.; Wang, G.; Guan, S.; Lu, J. Melatonin Prevents against Ethanol-Induced Liver Injury by Mitigating Ferroptosis via Targeting Brain and Muscle ARNT-like 1 in Mice Liver and HepG2 Cells. J. Agric. Food Chem. 2022, 70, 12953–12967. [Google Scholar] [CrossRef] [PubMed]
- Kvetnoy, I.; Ivanov, D.; Mironova, E.; Evsyukova, I.; Nasyrov, R.; Kvetnaia, T.; Polyakova, V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. Int. J. Mol. Sci. 2022, 23, 1835. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a Potential Therapy for Sepsis: A Phase I Dose Escalation Study and an Ex Vivo Whole Blood Model under Conditions of Sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Maria, S.; Witt-Enderby, P.A. Melatonin Effects on Bone: Potential Use for the Prevention and Treatment for Osteopenia, Osteoporosis, and Periodontal Disease and for Use in Bone-Grafting Procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, F.L. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The Reduction in Circulating Levels of Melatonin May Be Associated with the Development of Preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671. [Google Scholar] [CrossRef]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, M.; Karandish, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Mousavi, R. Metabolic and Hormonal Effects of Melatonin and/or Magnesium Supplementation in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutr. Metab. 2021, 18, 57. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; José Maria Soares, J.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Gonçalves, I.; Santos, C.R.A.; Quintela, T. The Crosstalk between Melatonin and Sex Steroid Hormones. Neuroendocrinology 2022, 112, 115–129. [Google Scholar] [CrossRef]
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of Melatonin Supplementation on Sleep Quality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2022, 269, 205–216. [Google Scholar] [CrossRef]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A Pleiotropic Molecule Regulating Inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlitz, M.; Alvarez, B.; Vignau, J.; English, J.; Arendt, J.; Parkes, J. Delayed Sleep Phase Syndrome Response to Melatonin. Lancet 1991, 337, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.M.; Gooley, J.J.; Saper, C.B. Neurobiology of the Sleep-Wake Cycle: Sleep Architecture, Circadian Regulation, and Regulatory Feedback. J. Biol. Rhythm. 2006, 21, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Reiter, R.J.; Wasdell, M.B.; Bax, M. The Role of the Thalamus in Sleep, Pineal Melatonin Production, and Circadian Rhythm Sleep Disorders. J. Pineal Res. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; D’Mello, J., Ed.; CAB Intern: Boston, MA, USA, 2015; pp. 390–435. ISBN 978-1-78064-263-5. [Google Scholar]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the Efficacy of Melatonin in the Treatment of Primary Adult Sleep Disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Amaral, F.; Silva, J.-A.; Kuwabara, W.; Cipolla-Neto, J. New Insights into the Function of Melatonin and Its Role in Metabolic Disturbances. Expert Rev. Endocrinol. Metabol. 2019, 14, 293–300. [Google Scholar] [CrossRef]
- Waterhouse, J.; Reilly, T.; Atkinson, G. Jet Lag. Lancet 1997, 350, 1611–1616. [Google Scholar] [CrossRef]
- Takahashi, T.; Sasaki, M.; Itoh, H.; Ozone, M.; Yamadera, W.; Hayshida, K.I.; Ushijima, S.; Matsunaga, N.; Obuchi, K.; Sano, H. Effect of 3 Mg Melatonin on Jet Lag Syndrome in an 8-h Eastward Flight. Psychiatry Clin. Neurosci. 2000, 54, 377–378. [Google Scholar] [CrossRef]
- Takahashi, T.; Sasaki, M.; Itoh, H.; Yamadera, W.; Ozone, M.; Obuchi, K.; Hayashida, K.I.; Matsunaga, N.; Sano, H. Melatonin Alleviates Jet Lag Symptoms Caused by an 11-Hour Eastward Flight. Psychiatry Clin. Neurosci. 2002, 56, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Herxheimer, A. Jet Lag. Clin. Evid 2005, 13, 2178–2183. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin During Postharvest of Horticultural Crops. Plant Cell Physiol. 2021, pcab175. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. The Multi-Regulatory Properties of Melatonin in Plants. In Neurotransmitters in Plants; Ramakrishna, A., Roshchina, V.V., Eds.; CRC Press: Boca Raton, FL, USA, 2018; p. 448. ISBN 978-0-203-71148-4. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Regulatory Role of Melatonin in the Redox Network of Plants and Plant Hormone Relationship in Stress. In Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Plant in Challenging Environments; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–272. ISBN 978-3-030-77477-6. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2022, 22, 413–429. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin Is a Potential Target for Improving Horticultural Crop Resistance to Abiotic Stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile Roles of Melatonin in Growth and Stress Tolerance in Plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Yang, X.; Ren, J.; Li, J.; Lin, X.; Xia, X.; Yan, W.; Zhang, Y.; Deng, X.; Ke, Q. Meta-Analysis of the Effect of Melatonin Application on Abiotic Stress Tolerance in Plants. Plant Biotechnol. Rep. 2022. [Google Scholar] [CrossRef]
- Sati, H.; Khandelwal, A.; Pareek, S. Effect of Exogenous Melatonin in Fruit Postharvest, Crosstalk with Hormones, and Defense Mechanism for Oxidative Stress Management. Food Front. 2023, 1–29. [Google Scholar] [CrossRef]
- Ahmad, S. Interactive Effects of Melatonin and Nitrogen Improve Drought Tolerance of Maize Seedlings by Regulating Growth and Physiochemical Attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative Effect of Melatonin Improves Drought Tolerance by Regulating Growth, Photosynthetic Traits and Leaf Ultrastructure of Maize Seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, C.L.; Esteban-Zubero, E.; Zhou, Z.; Reiter, J.R. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic Acid O-Methyltransferase Is Involved in the Synthesis of Melatonin by Methylating N-Acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis thaliana Plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.Y.; Back, K. Rice Histone Deacetylase 10 and Arabidopsis Histone Deacetylase 14 Genes Encode N-Acetylserotonin Deacetylase, Which Catalyzes Conversion of N-Acetylserotonin into Serotonin, a Reverse Reaction for Melatonin Biosynthesis in Plants. J. Pineal Res. 2018, 64, e12460. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.-J.; Back, K. Functional Characterization of Arylalkylamine N-Acetyltransferase, a Pivotal Gene in Antioxidant Melatonin Biosynthesis from Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.O.; Back, K. Simultaneous Suppression of Two Distinct Serotonin N-Acetyltransferase Isogenes by RNA Interference Leads to Severe Decreases in Melatonin and Accelerated Seed Deterioration in Rice. Biomolecules 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Lee, K.; Back, K. Knockout of Arabidopsis Serotonin N-Acetyltransferase-2 Reduces Melatonin Levels and Delays Flowering. Biomolecules 2019, 9, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An Evolutionary View of Melatonin Synthesis and Metabolism Related to Its Biological Functions in Plants. J. Exp. Bot 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Danilovich, M.E.; Alberto, M.R.; Juárez Tomás, M.S. Microbial Production of Beneficial Indoleamines (Serotonin and Melatonin) with Potential Application to Biotechnological Products for Human Health. J. Appl. Microbiol. 2021, 131, 1668–1682. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Al-Awadi, S.; Oommen, S.; Alkhamis, A.; Afzal, M. Tryptophan Oxidative Metabolism Catalyzed by Geobacillus stearothermophilus: A Thermophile Isolated from Kuwait Soil Contaminated with Petroleum Hydrocarbons. Int. J. Tryptophan Res. 2011, 4, IJTR.S6457. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Validation of an Analytical Method to Determine Melatonin and Compounds Related to L-Tryptophan Metabolism Using UHPLC/HRMS. Food Anal. Methods 2016, 9, 3327–3336. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant. Sci. 2016, 7, 1387. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic Bacterium Pseudomonas fluorescens RG11 May Transform Tryptophan to Melatonin and Promote Endogenous Melatonin Levels in the Roots of Four Grape Cultivars. Front. Plant Sci. 2017, 7, 2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñiz-Calvo, S.; Bisquert, R.; Guillamón, J.M. Melatonin in Yeast and Fermented Beverages: Analytical Tools for Detection, Physiological Role and Biosynthesis. Melatonin Res. 2020, 3, 144–160. [Google Scholar] [CrossRef]
- Muñiz-Calvo, S.; Bisquert, R.; Fernández-Cruz, E.; García-Parrilla, M.C.; Guillamón, J.M. Deciphering the Melatonin Metabolism in Saccharomyces cerevisiae by the Bioconversion of Related Metabolites. J. Pineal Res. 2019, 66, e12554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.-Z. Melatonin and Other 5-Methoxylated Indoles in Yeast: Presence in High Concentrations and Dependence on Tryptophan Availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, M.d.C. Production of Melatonin by Saccharomyces Strains under Growth and Fermentation Conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-López, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic Fermentation Induces Melatonin Synthesis in Orange Juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin Immunoreactivity in the Photosynthetic Prokaryote Rhodospirillum rubrum: Implications for an Ancient Antioxidant System. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin Production in an Aerobic Photosynthetic Bacterium: An Evolutionarily Early Association with Darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Non-Vertebrate Melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Carrasco-Galán, F.; Cerezo-López, A.B.; Valero, E.; Morcillo-Parra, M.Á.; Beltran, G.; Torija, M.-J.; Troncoso, A.M.; García-Parrilla, M.C. Occurrence of Melatonin and Indolic Compounds Derived from L-Tryptophan Yeast Metabolism in Fermented Wort and Commercial Beers. Food Chem. 2020, 331, 127192. [Google Scholar] [CrossRef]
- Luo, H.; Förster, J. Optimized Microbial Cells for Production of Melatonin and Other Compounds. U.S. Patent US10851365B2, 5 October 2017. [Google Scholar]
- Luo, H.; Schneider, K.; Christensen, U.; Lei, Y.; Herrgard, M.; Palsson, B.Ø. Microbial Synthesis of Human-Hormone Melatonin at Gram Scales. ACS Synth. Biol. 2020, 9, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Valero, N.; Chacin-Bonilla, L.; Medina-Leendertz, S. Melatonin and Viral Infections. J. Pineal Res. 2004, 36, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. Urinary 6-Sulphatoxymelatonin Excretory Rhythms in Laboratory Rats: Effects of Photoperiod and Light. Brain Res. 1993, 603, 338–342. [Google Scholar] [CrossRef]
- Hugel, H.M.; Kennaway, D.J. Synthesis and Chemistry of Melatonin and of Related Compounds. A Review. Org. Prep. Proced. Int. 1995, 27, 1–31. [Google Scholar] [CrossRef]
- Williamson, B.L.; Tomlinson, A.J.; Mishra, P.K.; Gleich, G.J.; Naylor, S. Structural Characterization of Contaminants Found in Commercial Preparations of Melatonin: Similarities to Case-Related Compounds from L-Tryptophan Associated with Eosinophilia-Myalgia Syndrome. Chem. Res. Toxicol 1998, 11, 234–240. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef]
- Williamson, B.L.; Kenneth, L.J.; Tomlinson, A.J.; Gleich, G.J.; Naylor, S. On-Line HPLC-Tandem Mass Spectrometry Structural Characterization of Case Associated Contaminants of L-Tryptophan Implicated with the Onset of Eosinophilia-Myalgia Syndrome. Toxicol. Lett. 1988, 99, 139–150. [Google Scholar] [CrossRef]
- Williamson, B.L.; Tomlinson, A.J.; Naylor, S.; Gleich, G.J. Contaminats in Commercial Preparations of Melatonin. Mayo Clin. Proceed. 1997, 72, 1094–1095. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Li, J.L.; Zhang, J.J.; Su, P.; Zheng, S.L. Microwave Assisted Synthesis of Melatonin. Synth. Commun. 2003, 33, 741–747. [Google Scholar] [CrossRef]
- OECD-Organisation for Economic Co-operation & Development. Initial Assessment Report on Phthalimide; ID-85-41-6; SIAM 20; Screening Information DataSet (SIDS): Paris, France, 2006. [Google Scholar]
- Verspui, G.; Elbertse, G.; Sheldon, F.A.; Hacking, M.A.P.J.; Sheldon, R.A. Selective Hydroformylation of N-Allylacetamide in an Inverted Aqueous Two-Phase Catalytic System, Enabling a Short Synthesis of Melatonin. Chem. Commun. 2000, 2000, 1363–1364. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin: Searching for Plants with High Levels as a Natural Source of Nutraceuticals. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, F.R.S., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2015; Volume 46, pp. 519–545. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.S.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The Changing Biological Roles of Melatonin during Evolution: From an Antioxidat to Signals of Darkness, Sexual Selection and Fitness. Biol. Rev. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, e815769. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in the Evolution of Plants and Other Phototrophs. Melatonin Res. 2019, 2, 10–36. [Google Scholar] [CrossRef]
- Germann, S.M.; Baallal Jacobsen, S.A.; Schneider, K.; Harrison, S.J.; Jensen, N.B.; Chen, X.; Stahlhut, S.G.; Borodina, I.; Luo, H.; Zhu, J.; et al. Glucose-Based Microbial Production of the Hormone Melatonin in Yeast Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Chen, L.; Zhang, W. Microbial Production of Mammalian Melatonin—A Promising Solution to Melatonin Industry. Biotechnol. J. 2016, 11, 601–602. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Reiter, R.J. Emergence of Naturally Occurring Melatonin Isomers and Their Proposed Nomenclature. J. Pineal Res. 2012, 53, 113–121. [Google Scholar] [CrossRef]
- Arnao, M.B.; Castejón, A.; Giraldo-Acosta; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin as an Alternative to Synthetic Melatonin: Content in Some Aromatic Plants of Interest; SEFIT: Sant Joan d’Alacant, Spain, 2021. [Google Scholar]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants. Possible Function in Germ Tissue Protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Marioni, F.; Bertoli, A.; Pistelli, L. A Straightforward Procedure to Biosynthesis Melatonin Using Freshly Chopped Achillea millefolium L. as Reagent. Phytochem. Lett. 2008, 1, 107–110. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Szwajgier, D.; Gawel-Beben, K.; Strzepek-Gomolka, M.; Glowniak, K.; Meissner, H.O. Is Phytomelatonin Complex Better Than Synthetic Melatonin? The Assessment of the Antiradical and Anti-Inflammatory Properties. Molecules 2021, 26, 6087. [Google Scholar] [CrossRef]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality Analysis of Commercial Chlorella Products Used as Dietary Supplement in Human Nutrition. J. Appl. Phycol. 2010, 22, 265–276. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and Quantification of the Antioxidant Melatonin in Montmorency and Balaton Tart Cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Barbero, G.F.; Palma, M.; García Barroso, C. Determination of Melatonin in Rice (Oryza sativa) Grains by Pressurized Liquid Extraction. J. Agric. Food Chem. 2015, 63, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Duros, E.; Palma, M.; Barroso, C.G. Optimization of the Ultrasound-Assisted Extraction of Melatonin from Red Rice (Oryza sativa) Grains through a Response Surface Methodology. Appl. Acoust. 2016, 103, 129–135. [Google Scholar] [CrossRef]
- Setyaningsih, W.; García, K.G.; Rodríguez, M.C.; Palma, M.; Barroso, C. Towards Healthy Products Elaborated with Melatonin-Rich Rices. Investig. Y Desarro. En Cienc. Y Tecnol. De Aliment. 2016, 1, 77–86. [Google Scholar]
- Chakraborty, S.; Bhattacharjee, P. Supercritical Carbon Dioxide Extraction of Melatonin from Brassica campestris: In Vitro Antioxidant, Hypocholesterolemic and Hypoglycaemic Activities of the Extracts. Int. J. Pharm. Sci. Res. 2017, 8, 2486–2495. [Google Scholar]
- Chakraborty, S.; Bhattacharjee, P. Ultrasonication-Assisted Extraction of a Phytomelatonin-Rich, Erucic Acid-Lean Nutraceutical Supplement from Mustard Seeds: An Antioxidant Synergy in the Extract by Reductionism. J. Food Sci. Technol. 2020, 57, 1278–1289. [Google Scholar] [CrossRef]
- Losada, M.; Cano, A.; Hernández-Ruïz, J.; Arnao, M.B. Phytomelatonin Content in Valeriana officinalis L. and Some Related Phytotherapeutic Supplements. Int. J. Plant Based Pharm. 2022, 2, 176–181. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin, Natural Melatonin from Plants as a Novel Dietary Supplement: Sources, Activities and World Market. J. Funct. Foods 2018, 48, 37–42. [Google Scholar] [CrossRef]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Ma, T.; Deng, Z.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-Derived Melatonin from Food: A Gift of Nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Espino, J.; González-Gómez, D.; Lozano, M.; Cubero, J.; Toribio-Delgado, A.F.; Maynar-Mariño, J.I.; Terrón, M.P.; Muñoz, J.L.; Pariente, J.A.; et al. A Nutraceutical Product Based on Jerte Valley Cherries Improves Sleep and Augments the Antioxidant Status in Humans. Eur. J. Clin. Nutr. Metab. 2009, 4, e321–e323. [Google Scholar] [CrossRef] [Green Version]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley Cherry-Enriched Diets Improve Nocturnal Rest and Increase 6-Sulfatoxymelatonin and Total Antioxidant Capacity in the Urine of Middle-Aged and Elderly Humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef] [PubMed]



| Common Name | Scientific Name | Organ or Zone | Phytomelatonin Content (ng/g DW ± SE) |
|---|---|---|---|
| Thyme-1 | Thymus vulgaris | Leaf (L) | 77.8 ± 3.8 |
| Thyme-2 | 134.1 ± 9.3 | ||
| Thyme-3 | 1419.5 ± 71.5 | ||
| Thyme-4 | 625.3 ± 60.6 | ||
| 26–3000 * | |||
| Lemon thyme-1 | Thymus citriodorus | L | 154.0 ± 10.1 |
| Lemon thyme-2 | 243.8 ± 13.9 | ||
| Red thyme-1 | Thymus zigis | L | 158.9 ± 9.9 |
| Red thyme-2 | 306.9 ± 25.4 | ||
| Valerian-1 | Valeriana officinalis | Root (R) | 1510.3 ± 83.2 |
| Valerian-2 | 2060.8 ± 125.5 | ||
| Valerian-3 | 60.7 ± 4.5 | ||
| Valerian-4 | 180.5 ± 15.2 | ||
| Valerian-5 | 455.0 ± 27.6 | ||
| Valerian-6 | 745.9 ± 55.3 | ||
| 80–300 * | |||
| Gentian-1 | Gentiana lutea | R | 222.9 ± 11.5 |
| Gentian-2 | 113.7 ± 6.6 | ||
| 180–300 * | |||
| Sage-1 | Salvia officinalis | Entire(E) | 17.2 ± 0.8 |
| Sage-2 | L | 146.5 ± 7.7 | |
| Sage-3 | 223.6 ± 18.5 | ||
| 32–29,000 * | |||
| Spanish sage | Salvia lavanducifolia | L | 136.9 ± 10.6 |
| Chamomille-1 | Matricaria chamomilla | L | 61.3 ± 2.6 |
| Chamomille-2 | 95.4 ± 5.1 | ||
| Lemon balm mint-1 | Melissa officinalis | L | 18.5 ± 1.3 |
| Lemon balm mint-2 | 11.4 ± 1.0 | ||
| Lemon balm mint-3 | 26.8 ± 2.1 | ||
| 50–100 * | |||
| Cat’s claw-1 | Uncaria tomentosa | E | 64.5 ± 5.5 |
| Cat’s claw-2 | 72.9 ± 6.9 | ||
| Uncaria rhynchophylla | 2460 * | ||
| Lemon verbena-1 | Aloysia citriodora | L | 239.4 ± 18.3 |
| Lemon verbena-2 | 250.9 ± 20.2 | ||
| 1200 * | |||
| St. John’s Wort-1 | Hypericum perforatum | R | 3650.7 ± 201.6 |
| St. John’s Wort-2 | 1500.3 ± 122.3 | ||
| St. John’s Wort-3 | 2265.8 ± 138.9 | ||
| 11–23,000 * | |||
| Harpagophyte | Harpagophytum procumbens | R | 16.8 ± 1.2 |
| MAP-1 | Different species | L | 25–125 |
| 1800–10,000 (elicited) | |||
| MAP-2 | Different species | L | 50–230 |
| 3200–20,000 (elicited) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites 2023, 13, 72. https://doi.org/10.3390/metabo13010072
Arnao MB, Giraldo-Acosta M, Castejón-Castillejo A, Losada-Lorán M, Sánchez-Herrerías P, El Mihyaoui A, Cano A, Hernández-Ruiz J. Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites. 2023; 13(1):72. https://doi.org/10.3390/metabo13010072
Chicago/Turabian StyleArnao, Marino B., Manuela Giraldo-Acosta, Ana Castejón-Castillejo, Marta Losada-Lorán, Pablo Sánchez-Herrerías, Amina El Mihyaoui, Antonio Cano, and Josefa Hernández-Ruiz. 2023. "Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin" Metabolites 13, no. 1: 72. https://doi.org/10.3390/metabo13010072
APA StyleArnao, M. B., Giraldo-Acosta, M., Castejón-Castillejo, A., Losada-Lorán, M., Sánchez-Herrerías, P., El Mihyaoui, A., Cano, A., & Hernández-Ruiz, J. (2023). Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites, 13(1), 72. https://doi.org/10.3390/metabo13010072