Plasma-Sputtered Growth of Ni-Pd Bimetallic Nanoparticles on Carbon Nanotubes for Toluene Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bimetallic NPs Decoration of MWCNTs
2.2. Characterization
2.3. Gas Sensing
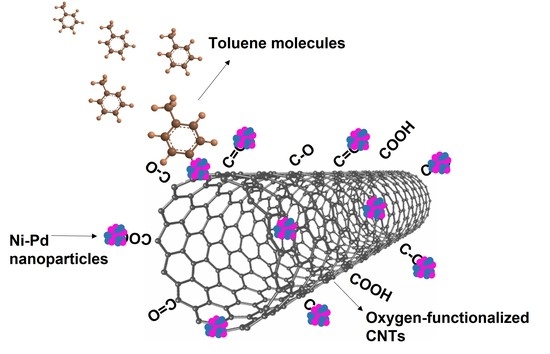
3. Results and Discussion

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ma, Z.; Ma, J.; Yang, L.; Wei, J.; Zhao, Y.; Zhang, M.; Yang, F.; Wang, X. Recent Progress of Miniature MEMS Pressure Sensors. Micromachines 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhar, I.; Mandal, N. A Review on Advanced Wireless Passive Temperature Sensors. Measurement 2022, 187, 110255. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Young, S.-J.; Liu, Y.-H.; Lin, Z.-D.; Ahmed, K.; Shiblee, M.N.I.; Romanuik, S.; Sekhar, P.K.; Thundat, T.; Nagahara, L.; Arya, S.; et al. Multi-Walled Carbon Nanotubes Decorated with Silver Nanoparticles for Acetone Gas Sensing at Room Temperature. J. Electrochem. Soc. 2020, 167, 167519. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N. A Review on Chemiresistive Gas Sensors Based on Carbon Nanotubes: Device and Technology Transformation. Sens. Actuators A Phys. 2018, 283, 174–186. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Adeel, M.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Carbon Nanotubes-Based Cues: A Pathway to Future Sensing and Detection of Hazardous Pollutants. J. Mol. Liq. 2019, 292, 111425. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Jin, W.; Hu, Y.; Cui, Y. Carbon Nanotube Field-Effect Transistor-Based Chemical and Biological Sensors. Sensors 2021, 21, 995. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Hua, C.; Shang, Y.; Wang, Y.; Xu, J.; Zhang, Y.; Li, X.; Cao, A. A Flexible Gas Sensor Based on Single-Walled Carbon Nanotube-Fe2O3 Composite Film. Appl. Surf. Sci. 2017, 405, 405–411. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernández, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon Nanotube Networks as Gas Sensors for NO2 Detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Naje, A.N.; Ibraheem, R.R.; Ibrahim, F.T. Parametric Analysis of NO2 Gas Sensor Based on Carbon Nanotubes. Photonic Sens. 2016, 6, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Nag, A.; Chandra Mukhopadhyay, S.; Xu, Y. Carbon Nanotubes and Its Gas-Sensing Applications: A Review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H.; Pourfath, M.; Moradi, M. High Sensitive and Selective Flexible H2S Gas Sensors Based on Cu Nanoparticle Decorated SWCNTs. Sens. Actuators B Chem. 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One Hundred Fold Increase in Current Carrying Capacity in a Carbon Nanotube–Copper Composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef] [Green Version]
- Panzer, M.A.; Zhang, G.; Mann, D.; Hu, X.; Pop, E.; Dai, H.; Goodson, K.E. Thermal Properties of Metal-Coated Vertically Aligned Single-Wall Nanotube Arrays. J. Heat Transfer. 2008, 130, 052401. [Google Scholar] [CrossRef] [Green Version]
- Cross, R.; Cola, B.A.; Fisher, T.; Xu, X.; Gall, K.; Graham, S. A Metallization and Bonding Approach for High Performance Carbon Nanotube Thermal Materials. Nanotechnology 2010, 21, 445705. [Google Scholar] [CrossRef]
- Bult, J.; Sawyer, W.G.; Voevodin, A.; Muratore, C.; Dickrell, P.; Pal, S.; Ajayan, P.; Schadler, L. Electrical Switching Using Compliant Metal Infiltrated Multi-Wall Nanotube Arrays. MRS Online Proc. Libr. 2008, 1085, 1085-T02-05. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Application of Metal Nanoparticles Decorated Carbon Nanotubes in Photovoltaics. Appl. Phys. Lett. 2008, 93, 033315. [Google Scholar] [CrossRef]
- Claussen, J.C.; Franklin, A.D.; Haque, A.U.; Marshall Porterfield, D.; Fisher, T.S. Electrochemical Biosensor of Nanocube-Augmented Carbon Nanotube Networks. ACS Nano 2009, 3, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal Nanoparticles and Related Materials Supported on Carbon Nanotubes: Methods and Applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Koziol, K.K.K. The Influence of Metal Nanoparticles on Electrical Properties of Carbon Nanotubes. Appl. Surf. Sci. 2016, 376, 74–78. [Google Scholar] [CrossRef]
- Rabat, H.; Andreazza, C.; Brault, P.; Caillard, A.; Béguin, F.; Charles, C.; Boswell, R. Carbon/Platinum Nanotextured Films Produced by Plasma Sputtering. Carbon 2009, 47, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, Y.; Yang, L.; Chen, S.; Weng, J.; Yue, L. Polymer Driven Covalently Bonded Decahedral-Twinning of Ag Nanoparticles Prepared by ICP Enhanced Magnetron Sputtering Method. J. Phys. Chem. C 2009, 113, 7633–7638. [Google Scholar] [CrossRef]
- Window, B. Recent Advances in Sputter Deposition. Surf. Coat. Technol. 1995, 71, 93–97. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S.; Greene, J.E. Sputter Deposition Processes. In Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 253–296. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Franklin, N.W.; Chen, R.J.; Dai, H. Metal Coating on Suspended Carbon Nanotubes and Its Implication to Metal–Tube Interaction. Chem. Phys. Lett. 2000, 331, 35–41. [Google Scholar] [CrossRef]
- Fang, Y.L.; Miller, J.T.; Guo, N.; Heck, K.N.; Alvarez, P.J.J.; Wong, M.S. Structural Analysis of Palladium-Decorated Gold Nanoparticles as Colloidal Bimetallic Catalysts. Catal. Today 2011, 160, 96–102. [Google Scholar] [CrossRef]
- Felten, A.; Ghijsen, J.; Pireaux, J.-J.; Drube, W.; Johnson, R.L.; Liang, D.; Hecq, M.; van Tendeloo, G.; Bittencourt, C. Electronic Structure of Pd Nanoparticles on Carbon Nanotubes. Micron 2009, 40, 74–79. [Google Scholar] [CrossRef]
- Lobo, A.O.; Ramos, S.C.; Antunes, E.F.; Marciano, F.R.; Trava-Airoldi, V.J.; Corat, E.J. Fast Functionalization of Vertically Aligned Multiwalled Carbon Nanotubes Using Oxygen Plasma. Mater. Lett. 2012, 70, 89–93. [Google Scholar] [CrossRef]
- Acosta, S.; Casanova Chafer, J.; Sierra Castillo, A.; Llobet, E.; Snyders, R.; Colomer, J.-F.; Quintana, M.; Ewels, C.; Bittencourt, C. Low Kinetic Energy Oxygen Ion Irradiation of Vertically Aligned Carbon Nanotubes. Appl. Sci. 2019, 9, 5342. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, C.; Navio, C.; Nicolay, A.; Ruelle, B.; Godfroid, T.; Snyders, R.; Colomer, J.-F.; Lagos, M.J.; Ke, X.; van Tendeloo, G.; et al. Atomic Oxygen Functionalization of Vertically Aligned Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 20412–20418. [Google Scholar] [CrossRef]
- Han, Y.-F.; Kumar, D.; Sivadinarayana, C.; Clearfield, A.; Goodman, D.W. The Formation of PdCx over Pd-Based Catalysts in Vapor-Phase Vinyl Acetate Synthesis: Does a Pd–Au Alloy Catalyst Resist Carbide Formation? Catal. Lett. 2004, 94, 131–134. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Bradshaw, A.M.; Guo, Q.; Goodman, D.W. The Size Dependence of the Electronic Structure of Pd Clusters Supported on Al2O3/Re(0001). Surf. Sci. 1998, 399, L357–L363. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Bahena Uribe, D.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic Hydrogen Evolution Using Ni–Pd/TiO2: Correlation of Light Absorption, Charge-Carrier Dynamics, and Quantum Efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Xiong, D.; Li, W.; Liu, L. Vertically Aligned Porous Nickel(II) Hydroxide Nanosheets Supported on Carbon Paper with Long-Term Oxygen Evolution Performance. Chem. Asian J. 2017, 12, 543–551. [Google Scholar] [CrossRef]
- Cheng, M.; Fan, H.; Song, Y.; Cui, Y.; Wang, R. Interconnected Hierarchical NiCo2O4 Microspheres as High-Performance Electrode Materials for Supercapacitors. Dalton Trans. 2017, 46, 9201–9209. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect Characterization in Graphene and Carbon Nanotubes Using Raman Spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.J.; Na, H.G.; Kang, S.Y.; Choi, S.-W.; Kim, S.S.; Kim, H.W. Selective Detection of Low Concentration Toluene Gas Using Pt-Decorated Carbon Nanotubes Sensors. Sens. Actuators B Chem. 2016, 227, 157–168. [Google Scholar] [CrossRef]
- Clément, P.; Korom, S.; Struzzi, C.; Parra, E.J.; Bittencourt, C.; Ballester, P.; Llobet, E. Bezene Detection: Deep Cavitand Self-Assembled on Au NPs-MWCNT as Highly Sensitive Benzene Sensing Interface. Adv. Funct. Mater. 2015, 25, 4172. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Li, Z.-L.; Peng, Z.; Yang, J.-H.; Liu, B.; Liu, Z. Bimetallic Pd-M (M = Pt, Ni, Cu, Co) nanoparticles catalysts with strong electrostatic metal-support interaction for hydrogenation of toluene and benzene. Mol. Catal. 2020, 492, 110992. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, S.; Casanova-Chafer, J.; Llobet, E.; Hemberg, A.; Quintana, M.; Bittencourt, C. Plasma-Sputtered Growth of Ni-Pd Bimetallic Nanoparticles on Carbon Nanotubes for Toluene Sensing. Chemosensors 2023, 11, 328. https://doi.org/10.3390/chemosensors11060328
Acosta S, Casanova-Chafer J, Llobet E, Hemberg A, Quintana M, Bittencourt C. Plasma-Sputtered Growth of Ni-Pd Bimetallic Nanoparticles on Carbon Nanotubes for Toluene Sensing. Chemosensors. 2023; 11(6):328. https://doi.org/10.3390/chemosensors11060328
Chicago/Turabian StyleAcosta, Selene, Juan Casanova-Chafer, Eduard Llobet, Axel Hemberg, Mildred Quintana, and Carla Bittencourt. 2023. "Plasma-Sputtered Growth of Ni-Pd Bimetallic Nanoparticles on Carbon Nanotubes for Toluene Sensing" Chemosensors 11, no. 6: 328. https://doi.org/10.3390/chemosensors11060328
APA StyleAcosta, S., Casanova-Chafer, J., Llobet, E., Hemberg, A., Quintana, M., & Bittencourt, C. (2023). Plasma-Sputtered Growth of Ni-Pd Bimetallic Nanoparticles on Carbon Nanotubes for Toluene Sensing. Chemosensors, 11(6), 328. https://doi.org/10.3390/chemosensors11060328