The Application of Hyperspectral Images in the Classification of Fresh Leaves’ Maturity for Flue-Curing Tobacco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
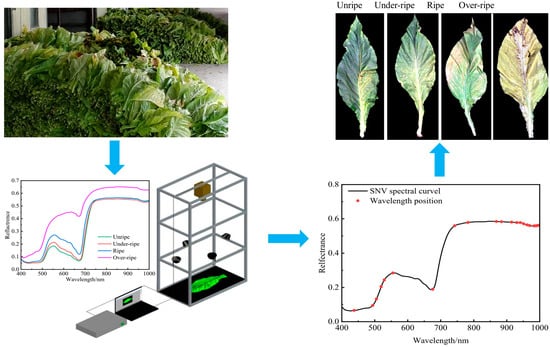
2.2. Acquisition of Hyperspectral Images
2.3. Hyperspectral Image Correction
2.4. Extraction of Spectral and Image Data
2.5. Data Analysis
2.6. Analytical Software
3. Results
3.1. Image Analysis
3.2. Spectra Analysis
3.3. Filter Processing of Images
3.4. PLS-DA of Tobacco Leaf Images
3.5. Selection of Characteristic Spectral Wavelengths
3.5.1. De-Trending
3.5.2. Standard Normalization Variable (SNV)
3.5.3. Multiple Scattering Correction (MSC)
3.6. Classification Results Using the Characteristic Wavelengths
3.7. Classification Results of Tobacco Leaves of Different Maturities Using SVN-SPA-PLS-DA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, F.L.; Liu, X.W.; Du, Z.G.; Hou, H.; Wang, X.Y.; Wang, F.L.; Yang, J.G. Bayesian phylodynamic analysis reveals the dispersal patterns of tobacco mosaic virus in China. Virology 2019, 528, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.G.; Zhu, J.; Lu, X.P.; Bai, Y.F.; Li, Y.P. Analysis on genetic contribution of agronomic traits to total sugar in flue-cured tobacco (Nicotiana tabacum L.). Field Crops Res. 2007, 102, 98–103. [Google Scholar] [CrossRef]
- Yin, F.; Karangwa, E.; Song, S.Q.; Duhoranimana, E.; Lin, S.S.; Cui, H.P.; Zhang, X.M. Contribution of tobacco composition compounds to characteristic aroma of Chinese faint-scent cigarettes through chromatography analysis and partial least squares regression. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2019, 1105, 217–227. [Google Scholar] [CrossRef]
- Zou, C.M.; Li, Y.; Huang, W.; Zhao, G.K.; Pu, G.R.; Su, J.E.; Coyne, M.S.; Chen, Y.; Wang, L.C.; Hu, X.D.; et al. Rotation and manure amendment increase soil macro-aggregates and associated carbon and nitrogen stocks in flue-cured tobacco production. Geoderma 2018, 325, 49–58. [Google Scholar] [CrossRef]
- Chen, Y.; Bin, J.; Zou, C.M.; Ding, M.J. Discrimination of fresh tobacco leaves with different maturity levels by near-infrared (NIR) spectroscopy and deep learning. J. Anal. Methods Chem. 2021, 2021, 9912589. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.; Ekşioğlu, A.; Akdaş, E.Y. Transcript profiling of salt tolerant tobacco mutants generated via mutation breeding. Gene Expr. Patterns GEP 2018, 29, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.F.; Liu, G.S.; Liu, D.S.; Zhang, Y.Y.; Fan, W.G.; Xing, X.X. Comparison of different methods for estimating nitrogen concentration in flue-cured tobacco leaves based on hyperspectral reflectance. Field Crops Res. 2013, 150, 108–114. [Google Scholar] [CrossRef]
- Song, Z.P.; Li, T.S.; Zhang, Y.G.; Cao, H.J.; Gong, C.R.; Zhang, W.J. The mechanism of carotenoid degradation in flue-cured tobacco and changes in the related enzyme activities at the leaf-drying stage during the bulk curing process. Agric. Sci. China 2010, 9, 1381–1388. [Google Scholar] [CrossRef]
- Xiang, B.K.; Cheng, C.H.; Xia, J.; Tang, L.; Mu, J.R.; Bi, Y.M. Simultaneous identification of geographical origin and grade of flue-cured tobacco using NIR spectroscopy. Vib. Spectrosc. 2020, 111, 103182. [Google Scholar] [CrossRef]
- Li, J.X.; Zhao, H.; Zhu, S.P.; Huang, H.; Miao, Y.J.; Jiang, Z.Y. An improved lightweight network architecture for identifying tobacco leaf maturity based on deep learning. J. Intell. Fuzzy Syst. 2021, 41, 4149–4158. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ren, K.; Su, J.E.; He, X.; Zhao, G.K.; Hu, B.B.; Chen, Y.; Xu, Z.L.; Jin, Y.; Zou, C.M. Rotation and organic fertilizers stabilize soil water-stable aggregates and their associated carbon and nitrogen in flue-cured tobacco production. J. Soil Sci. Plant Nutr. 2020, 20, 192–205. [Google Scholar] [CrossRef]
- Çakir, R.; Çebi, U. The effect of irrigation scheduling and water stress on the maturity and chemical composition of Virginia tobacco leaf. Field Crops Res. 2010, 119, 269–276. [Google Scholar] [CrossRef]
- Diao, H.; Wu, Y.M.; Yang, Y.H.; Ouyang, J.; Li, J.H.; Lao, C.L.; Xu, X.Y. Study on the determination of the maturity level of tobacco leaf based on in-situ spectral measurement. Spectrosc. Spectr. Anal. 2016, 36, 1826–1830. [Google Scholar]
- Yu, C.X.; Ma, X.; Zhang, Y.H.; Li, J.H.; Zhao, L.L.; Xu, L.; Wen, Y.D.; Wang, Y.; Zhang, L.D. Tobacco plant parts similarity analysis based on near-infrared spectroscopy and SIMCA algorithm. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2011, 31, 924–927. [Google Scholar]
- Hu, H.J.; Pan, L.Q.; Sun, K.; Tu, S.C.; Sun, Y.; Wei, Y.Y.; Tu, K. Differentiation of deciduous-calyx and persistent-calyx pears using hyperspectral reflectance imaging and multivariate analysis. Comput. Electron. Agric. 2017, 137, 150–156. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Q.B.; Wang, B.J.; Lu, R.F. Analysis of hyperspectral scattering images using locally linear embedding algorithm for apple mealiness classification. Comput. Electron. Agric. 2012, 89, 175–181. [Google Scholar] [CrossRef]
- Naganathan, G.K.; Grimes, L.M.; Subbiah, J.; Calkins, C.R.; Samal, A.; Meyer, G.E. Visible/near-infrared hyperspectral imaging for beef tenderness prediction. Comput. Electron. Agric. 2008, 64, 225–233. [Google Scholar] [CrossRef]
- Steinbrener, J.; Posch, K.; Leitner, R. Hyperspectral fruit and vegetable classification using convolutional neural networks. Comput. Electron. Agric. 2019, 162, 364–372. [Google Scholar] [CrossRef]
- Yuan, R.R.; Liu, G.S.; He, J.G.; Wan, G.L.; Fan, N.Y.; Li, Y.; Sun, Y.R. Classification of Lingwu long jujube internal bruise over time based on visible near-infrared hyperspectral imaging combined with partial least squares-discriminant analysis. Comput. Electron. Agric. 2021, 182, 106043. [Google Scholar] [CrossRef]
- Ke, J.X.; Rao, L.B.; Zhou, L.M.; Chen, X.Y.; Zhang, Z.Q. Non-destructive determination of volatile oil and moisture content and discrimination of geographical origins of Zanthoxylum bungeanum Maxim. by hyperspectral imaging. Infrared Phys. Technol. 2020, 105, 103185. [Google Scholar] [CrossRef]
- Yang, W.; Nigon, T.; Hao, Z.Y.; Dias Paiao, G.D.; Fernández, F.G.; Mulla, D.; Yang, C. Estimation of corn yield based on hyperspectral imagery and convolutional neural network. Comput. Electron. Agric. 2021, 184, 106092. [Google Scholar] [CrossRef]
- Liu, K.H.; Yang, M.H.; Huang, S.T.; Lin, C.S. Plant species classification based on hyperspectral imaging via a lightweight convolutional neural network model. Front. Plant Sci. 2022, 13, 855660. [Google Scholar] [CrossRef]
- He, H.J.; Wang, Y.L.; Ou, X.Q.; Ma, H.J.; Liu, H.J.; Yan, J.H. Rapid determination of chemical compositions in chicken flesh by mining hyperspectral data. J. Food Compos. Anal. 2023, 116, 105069. [Google Scholar] [CrossRef]
- Rodriguez-Cobo, L.; Garcia-Allende, P.B.; Cobo, A.; Lopez-Higuera, J.M.; Conde, O.M. Raw material classification by means of hyperspectral imaging and hierarchical temporal memories. IEEE Sens. J. 2012, 12, 2767–2775. [Google Scholar] [CrossRef]
- Soares, F.L.F.; Marcelo, M.C.A.; Porte, L.M.F.; Pontes, O.F.S.; Kaiser, S. Inline simultaneous quantitation of tobacco chemical composition by infrared hyperspectral image associated with chemometrics. Microchem. J. 2019, 151, 104225. [Google Scholar] [CrossRef]
- Yang, C.; Lee, W.S.; Gader, P. Hyperspectral band selection for detecting different blueberry fruit maturity stages. Comput. Electron. Agric. 2014, 109, 23–31. [Google Scholar] [CrossRef]
- Yu, K.Q.; Zhao, Y.R.; Li, X.L.; Shao, Y.N.; Zhu, F.L.; He, Y. Identification of crack features in fresh jujube using Vis/NIR hyperspectral imaging combined with image processing. Comput. Electron. Agric. 2014, 103, 1–10. [Google Scholar] [CrossRef]
- He, H.J.; Wang, Y.L.; Wang, Y.Y.; Liu, H.J.; Zhang, M.; Ou, X.Q. Simultaneous quantifying and visualizing moisture, ash and protein distribution in sweet potato [Ipomoea batatas (L.) Lam] by NIR hyperspectral imaging. Food Chem. X 2023, 18, 100631. [Google Scholar] [CrossRef]
- Cai, J.Y.; Liang, M.; Wen, Y.D.; Yu, C.X.; Wang, L.P.; Wang, Y.; Zhao, L.L.; Li, J.H. Analysis of tobacco color and location features using visible-near infrared hyperspectral data. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2014, 34, 2758–2763. [Google Scholar]
- Shahadati-Moghaddam, Z.; Kahrizi, D.; Kazemi, E. Genetic characterization and molecular mapping of novel chlorophyll deficiency gene in air-cured tobacco (Nicotiana tabacum L.). Genetika 2017, 49, 77–86. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.C.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.; Massam, P.; Wright, L.; Zarco-Tejada, P.J. Using hyperspectral plant traits linked to photosynthetic efficiency to assess N and P partition. ISPRS J. Photogramm. Remote Sens. 2020, 169, 406–420. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Shi, Y.K.; Qin, Y.D.; Xuan, G.T.; Li, J.; Li, Q.K.; Yang, F.J.; Hu, Z.C. A new quantitative index for the assessment of tomato quality using Vis-NIR hyperspectral imaging. Food Chem. 2022, 386, 132864. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.M.; Chakraborty, S.K.; Mahanti, N.K.; Pandiselvam, R. Effect of germ orientation during Vis-NIR hyperspectral imaging for thedetection of fungal contamination in maize kernel using PLS-DA, ANN and 1D-CNN modelling. Food Control 2022, 139, 109007. [Google Scholar] [CrossRef]
- Wang, Z.L.; Fan, S.X.; Wu, J.Z.; Zhang, C.; Xu, F.Y.; Yang, X.H.; Li, J.B. Application of long-wave near infrared hyperspectral imaging for determination of moisture content of single maize seed. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Lu, C.H.; Zhang, Y.J.; Ju, W.; Wang, J.Z.; Hong, F.; Wang, T.; Ou, C.S. Moving-Window-Improved Monte Carlo Uninformative Variable Elimination Combining Successive Projections Algorithm for Near-Infrared Spectroscopy (NIRS). J. Spectrosc. 2020, 2020, 3590301. [Google Scholar] [CrossRef]
- He, H.J.; Chen, Y.; Li, G.L.; Wang, Y.Y.; Ou, X.Q.; Guo, J.L. Hyperspectral imaging combined with chemometrics for rapid detection of talcum powder adulterated in wheat flour. Food Control 2023, 144, 109378. [Google Scholar] [CrossRef]
- Zhao, H.S.; Bruzzone, L.; Guan, R.C.; Zhou, F.F.; Yang, C. Spectral spatial genetic algorithm-based unsupervised band selection for hyperspectral image classification. IEEE Trans. Geosci. Remote Sens. 2021, 59, 9616–9632. [Google Scholar] [CrossRef]
- Yu, W. Fuzzy modelling via on-line support vector machines. Int. J. Syst. Sci. 2010, 41, 1325–1335. [Google Scholar] [CrossRef]
- Chua, C.G.; Goh, A.T.C. A hybrid Bayesian back-propagation neural network approach to multivariate modelling. Int. J. Numer. Anal. Methods Geomech. 2003, 27, 651–667. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Liu, Y.X.; Chen, D.; Liu, L.; Li, C.B.; Kang, K.J.; Wang, L.Y.; He, Z.L.; Yang, X.E. Spatial variation and fractionation of fluoride in tobacco-planted soils and leaf fluoride concentration in tobacco in Bijie City, Southwest China. Environ. Sci. Pollut. Res. Int. 2021, 28, 26112–26123. [Google Scholar] [CrossRef]
- Huang, X.; Guan, H.D.; Bo, L.Y.; Xu, Z.Q.; Mao, X.M. Hyperspectral proximal sensing of leaf chlorophyll content of spring maize based on a hybrid of physically based modelling and ensemble stacking. Comput. Electron. Agric. 2023, 208, 107745. [Google Scholar] [CrossRef]
- Zhang, W.X.; Pan, L.; Lu, L.X. Prediction of TVB-N content in beef with packaging films using visible-near infrared hyperspectral imaging. Food Control 2023, 147, 109562. [Google Scholar] [CrossRef]














| Maturity Level | Samples (Piece) | Characteristics of Tobacco Leaf |
|---|---|---|
| Unripe | 36 | The leaves are dark green, the main veins and branches are green, the pubescence is obvious, and the angle between the stalk and leaves is less than 60°. |
| Under-ripe | 43 | The leaves are light green, the main vein is light green, the branch veins are all white, the pubescence is partially shed, and the angle between the stem and leaves is 60°~74°. |
| Ripe | 91 | The leaf surface is light yellow, the main vein and the branch veins are all white, the pubescence fell off, the leaf tip and leaf edge are whitish and rolled down, and the angle between the stem and leaves is 75°~85°. |
| Over-ripe | 53 | The complete leaf surface is yellow, the main veins and branches are white, the pubescence is completely shed, and the angle between the stem and leaves is greater than 85°. |
| Sample Set | Total | Unripe | Under-Ripe | Ripe | Over-Ripe |
|---|---|---|---|---|---|
| Calibration set | 148 | 25 | 32 | 57 | 34 |
| Prediction set | 65 | 11 | 11 | 24 | 19 |
| Pretreatment Method | Principal Components | Calibration Set (n = 148) | Prediction Set (n = 65) | ||
|---|---|---|---|---|---|
| Correct | Accuracy (%) | Correct | Accuracy (%) | ||
| Original | 14 | 130 | 87.84 | 60 | 92.31 |
| Baseline | 12 | 128 | 86.49 | 55 | 84.62 |
| De-trending | 19 | 136 | 91.89 | 61 | 93.85 |
| MSC | 16 | 141 | 95.27 | 63 | 96.92 |
| Normalize | 18 | 128 | 86.49 | 54 | 83.08 |
| OSC | 15 | 102 | 68.92 | 45 | 69.23 |
| SG | 12 | 129 | 87.16 | 55 | 84.62 |
| SNV | 13 | 137 | 92.57 | 61 | 93.85 |
| Spectroscopic | 11 | 126 | 85.14 | 52 | 80.00 |
| GF | 14 | 130 | 87.84 | 56 | 86.15 |
| MA | 13 | 129 | 87.16 | 56 | 86.15 |
| MF | 12 | 126 | 85.14 | 55 | 84.62 |
| Filter Method | Screening Method | Model | Calibration Set (n = 148) | Prediction Set (n = 65) | ||
|---|---|---|---|---|---|---|
| Correct | Accuracy (%) | Correct | Accuracy (%) | |||
| De-trending | SPA | GA | 132 | 89.19 | 59 | 90.77 |
| PLS-DA | 140 | 94.59 | 61 | 93.85 | ||
| LSVM | 115 | 77.70 | 52 | 80.00 | ||
| BPNN | 110 | 74.32 | 50 | 76.92 | ||
| PSO | GA | 116 | 78.38 | 50 | 76.92 | |
| PLS-DA | 136 | 91.89 | 59 | 90.77 | ||
| LSVM | 103 | 69.59 | 47 | 72.31 | ||
| BPNN | 108 | 72.97 | 49 | 75.38 | ||
| CARS | GA | 136 | 91.89 | 59 | 90.77 | |
| PLS-DA | 132 | 89.19 | 60 | 92.31 | ||
| LSVM | 116 | 78.38 | 52 | 80.00 | ||
| BPNN | 125 | 84.46 | 55 | 84.62 | ||
| SNV | SPA | GA | 141 | 95.27 | 63 | 96.92 |
| PLS-DA | 147 | 99.32 | 64 | 98.46 | ||
| LSVM | 138 | 93.24 | 59 | 90.77 | ||
| BPNN | 140 | 94.59 | 62 | 95.38 | ||
| PSO | GA | 132 | 89.19 | 57 | 87.69 | |
| PLS-DA | 143 | 96.62 | 61 | 93.85 | ||
| LSVM | 130 | 87.84 | 55 | 84.62 | ||
| BPNN | 131 | 88.51 | 56 | 86.15 | ||
| CARS | GA | 122 | 82.43 | 53 | 81.54 | |
| PLS-DA | 119 | 80.41 | 52 | 80.00 | ||
| LSVM | 130 | 87.84 | 59 | 90.77 | ||
| BPNN | 118 | 79.73 | 52 | 80.00 | ||
| MSC | SPA | GA | 137 | 92.57 | 56 | 86.15 |
| PLS-DA | 143 | 96.62 | 59 | 90.77 | ||
| LSVM | 135 | 91.22 | 56 | 86.15 | ||
| BPNN | 134 | 90.54 | 57 | 87.69 | ||
| PSO | GA | 139 | 93.92 | 52 | 80.00 | |
| PLS-DA | 145 | 97.97 | 62 | 95.38 | ||
| LSVM | 126 | 85.14 | 56 | 86.15 | ||
| BPNN | 121 | 81.76 | 55 | 84.62 | ||
| CARS | GA | 129 | 87.16 | 58 | 89.23 | |
| PLS-DA | 125 | 84.46 | 56 | 86.15 | ||
| LSVM | 105 | 70.95 | 47 | 72.31 | ||
| BPNN | 108 | 72.97 | 50 | 76.92 | ||
| Calibration Set (n = 148) | Accuracy (%) | Prediction Set (n = 65) | Accuracy (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unripe | Under-Ripe | Ripe | Over-Ripe | Unripe | Under-Ripe | Ripe | Over-Ripe | |||
| Unripe | 25 | 0 | 0 | 0 | 100 | 11 | 0 | 0 | 0 | 100 |
| Under-ripe | 0 | 32 | 0 | 0 | 100 | 0 | 11 | 0 | 0 | 100 |
| Ripe | 0 | 0 | 56 | 0 | 100 | 0 | 0 | 23 | 0 | 100 |
| Over-ripe | 0 | 0 | 1 | 34 | 97.14 | 0 | 0 | 1 | 19 | 95 |
| Accuracy/% | 100 | 100 | 98.24 | 100 | 99.32 | 100 | 100 | 95.83 | 100 | 98.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhao, C.; Qin, Y.; Xie, L.; Wang, T.; Wu, Z.; Xu, Z. The Application of Hyperspectral Images in the Classification of Fresh Leaves’ Maturity for Flue-Curing Tobacco. Processes 2023, 11, 1249. https://doi.org/10.3390/pr11041249
Lu X, Zhao C, Qin Y, Xie L, Wang T, Wu Z, Xu Z. The Application of Hyperspectral Images in the Classification of Fresh Leaves’ Maturity for Flue-Curing Tobacco. Processes. 2023; 11(4):1249. https://doi.org/10.3390/pr11041249
Chicago/Turabian StyleLu, Xiaochong, Chen Zhao, Yanqing Qin, Liangwen Xie, Tao Wang, Zhiyong Wu, and Zicheng Xu. 2023. "The Application of Hyperspectral Images in the Classification of Fresh Leaves’ Maturity for Flue-Curing Tobacco" Processes 11, no. 4: 1249. https://doi.org/10.3390/pr11041249
APA StyleLu, X., Zhao, C., Qin, Y., Xie, L., Wang, T., Wu, Z., & Xu, Z. (2023). The Application of Hyperspectral Images in the Classification of Fresh Leaves’ Maturity for Flue-Curing Tobacco. Processes, 11(4), 1249. https://doi.org/10.3390/pr11041249