Design and Development of Photocatalytic Systems for Reduction of CO2 into Valuable Chemicals and Fuels
Abstract
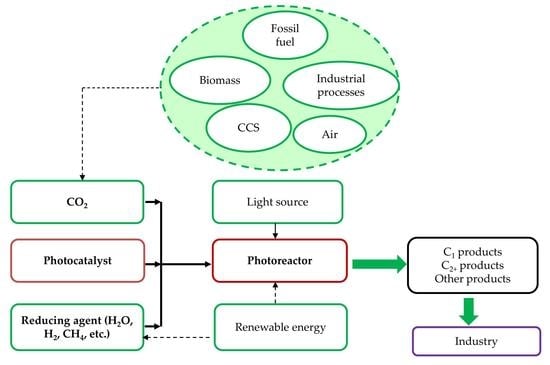
:1. Introduction
2. Fundamentals of CO2 Photoconversion
2.1. General Considerations
2.2. Modification of Existing Semiconductor Photocatalysts for Increasing Efficiency and Selectivity
2.3. Effect of Reductant and Sacrificial Agents on CO2 Photoreduction
2.4. Mechanism of the Photocatalytic CO2 Reduction
2.5. Photocatalysts for CO2 Reduction
2.5.1. TiO2-Based Photocatalysts
2.5.2. Indium Oxide
2.5.3. Cerium Oxide
2.5.4. Z-Scheme Heterostructures
2.5.5. MOF-Based Composites
2.5.6. Carbon Nitrides
2.5.7. Layered Double Hydroxides
2.6. Choice of Photocatalytic Supports
3. Photoreactor Design and Configuration
3.1. Slurry Photoreactors
3.2. Fixed Bed Photoreactors
3.3. Membrane Photoreactors
3.4. Solar Photoreactors
3.5. Modelling and Scale up of the Photoreactor
4. Challenges and Recommendations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- IEA. Global Energy Review: CO2 Emissions in 2021. International Energy Agency. March 2022. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 28 October 2022).
- Tiseo, I. Breakdown of CO2 Emissions in the EU-27 2020, by Sector. 2023. Available online: https://www.statista.com/statistics/1240108/road-transportation-greenhouse-gas-emissions-eu/?locale=en (accessed on 16 February 2023).
- US EPA. Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 28 October 2022).
- Albo, J.; Luis, P.; Irabien, A. Carbon dioxide capture from flue gases using a crossflow membrane contactor and the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Putting CO2 to Use: Creating Value from Emissions. International Energy Agency (IEA): Paris, France, 2019; p. 86. Available online: https://iea.blob.core.windows.net/assets/50652405-26db-4c41-82dc-c23657893059/Putting_CO2_to_Use.pdf (accessed on 16 February 2023).
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Choi, W.; Dong, F.; Hatzell, M. Solar Energy Utilization and Photo(electro)catalysis for Sustainable Environment. ACS EST Engg. 2022, 2, 940–941. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Ren, J.; Xu, R. Photocatalytic reduction of CO2: A brief review on product analysis and systematic methods. Anal. Methods 2013, 5, 1086–1097. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Z.; Wang, L. Photocatalytic and Photoelectrochemical Carbon Dioxide Reductions toward Value-Added Multicarbon Products. ACS EST Eng. 2022, 2, 975–988. [Google Scholar] [CrossRef]
- Levinson, R.; Berdahl, P.; Akbari, H. Solar Spectral Optical Properties of Pigments—Part I: Model for Deriving Scattering and Absorption Coefficients from Transmittance and Reflectance Measurements. Sol. Energy Mater. Sol. Cells 2005, 89, 319–349. [Google Scholar] [CrossRef]
- Kovacic, Z.; Likozar, B.; Hus, M. Photocatalytic CO2 reduction: A review of ab initio mechanism, kinetics, and multiscale modeling simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Hemminger, J.C.; Carr, R.; Somorjai, G.A. The photoassisted reaction of gaseous water and carbon dioxide adsorbed on the SrTiO3 (111) crystal face to form methane. Chem. Phys. Lett. 1978, 57, 100–104. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, S.; Woldu, A.R.; Hu, L. BiVO4/Bi2S3 Z-scheme heterojunction with MnOx as a cocatalyst for efficient photocatalytic CO2 conversion to methanol by pure water. Nano Energy 2022, 104, 107925. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Y.-X.; Tu, X.; Liu, C.-J. Nitrogen doping of indium oxide for enhanced photocatalytic reduction of CO2 to methanol. Nano Energy 2022, 101, 107613. [Google Scholar] [CrossRef]
- Shekar, G.C.S.; Alkanad, K.; Thejaswini, B.; Alnaggar, G.; Al Zaqri, N.; Drmosh, Q.; Boshaala, A.; Lokanath, N.K. Alkaline mediated sono-synthesis of surface oxygen-vacancies-rich cerium oxide for efficient photocatalytic CO2 reduction to methanol. Surf. Interfaces 2022, 34, 102389. [Google Scholar]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photoreactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Heng, H.; Gan, Q.; Meng, P.; Liu, X. H3PW12O40/TiO2-In2O3: A visible light driven type-II heterojunction photocatalyst for the photocatalytic degradation of imidacloprid. RSC Adv. 2016, 6, 73301. [Google Scholar] [CrossRef]
- Channei, D.; Chansaenpak, K.; Phanichphant, S.; Jannoey, P.; Khanitchaidecha, W.; Nakaruk, A. Synthesis and Characterization of WO3/CeO2 Heterostructured Nanoparticles for Photodegradation of Indigo Carmine Dye. ACS Omega 2021, 6, 19771–19777. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z. Study on light intensity in the process of photocatalytic degradation of indoor gaseous formaldehyde for saving energy. Energy Convers. Manag. 2007, 48, 882–889. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Rhimi, B.; Qin, J.; Huang, Y.; Yuan, M.; Wang, C. Oxygen-Doping of ZnIn2S4 Nanosheets Towards Boosted Photocatalytic CO2 Reduction. J. Energy Chem. 2021, 57, 1–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, Y.; Zhang, X.; Weinert, M.; Song, X.; Ai, J.; Han, L.; Fei, H. N-Heterocyclic Carbene-Stabilized Ultrasmall Gold Nanoclusters in a Metal-Organic Framework for Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17388–17393. [Google Scholar] [CrossRef]
- Li, D.; Hussain, S.; Wang, Y.; Huang, C.; Li, P.; Wang, M.; He, T. ZnSe/CdSe Z-Scheme Composites with Se Vacancy for Efficient Photocatalytic CO2 Reduction. Appl. Catal. B 2021, 286, 119887. [Google Scholar] [CrossRef]
- Tran, D.P.H.; Pham, M.-T.; Bui, X.-T.; Wang, Y.-F.; You, S.-J. CeO2 as a photocatalytic material for CO2 conversion: A review. Sol. Energy 2022, 240, 443–466. [Google Scholar] [CrossRef]
- Xia, X.H.; Jia, Z.H.; Yu, Y.; Liang, Y.; Wang, Z.; Ma, L.L. Preparation of multi-walled carbon nanotube supported TiO2 and its photocatalytic activity in the reduction of CO2 with H2O. Carbon 2007, 45, 717–721. [Google Scholar] [CrossRef]
- Ai, Z.H.; Ho, W.K.; Lee, S. Efficient visible light photocatalytic removal of NO with BiOBr–graphene nanocomposites. J. Phys. Chem. C 2011, 115, 25330–25337. [Google Scholar] [CrossRef]
- Woolerton, T.W.; Sheard, S.; Pierce, E.; Ragsdale, S.W.; Armstrong, F.A. CO2 photoreduction at enzyme-modified metal oxide nanoparticles. Energy Environ. L Sci. 2011, 4, 2393–2399. [Google Scholar] [CrossRef]
- Ettedgui, J.; Diskin-Posner, Y.; Weiner, L.; Neumann, R. Photoreduction of carbon dioxide to carbon monoxide with hydrogen catalyzed by a rhenium(I) phenanthroline–polyoxometalate hybrid complex. J. Am. Chem. Soc. 2010, 133, 188–190. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Bilal, M.; Hou, J.; Butt, F.K.; Ahmad, J.; Ali, S.; Hussain, A. Photocatalytic CO2 Reduction Using TiO2-Based Photocatalysts and TiO2 Z-Scheme Heterojunction Composites: A Review. Molecules 2022, 27, 2069. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 INTO Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation? Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Asakura, H.; Hosokawa, S.; Tanaka, T.; Teramura, K. Tuning Ag-modified NaTaO3 to achieve high CO selectivity for the photocatalytic conversion of CO2 using H2O as the electron donor. Appl. Catal. B Environ. 2023, 320, 121885. [Google Scholar] [CrossRef]
- Peng, C.; Reid, G.; Wang, H.; Hu, P. Perspective: Photocatalytic reduction of CO2 to solar fuels over semiconductors. J. Chem. Phys. 2017, 147, 030901. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Tawalbeh, M. Photocatalytic conversion of CO2 and H2O to useful fuels by nanostructured composite catalysis. Appl. Surf. Sci. 2019, 483, 363–372. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef]
- Olivo, A.; Ghedini, E.; Signoretto, M.; Compagnoni, M.; Rossetti, I. Liquid vs. Gas Phase CO2 Photoreduction Process: Which Is the Effect of the Reaction Medium? Energies 2017, 10, 1394. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Zhao, Z.; Tao, H.; Sun, Z. Heterogeneous catalysis of CO2 hydrogenation to C2+ products. Acta Phys.-Chim. Sin. 2018, 34, 858–872. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RCS Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Xu, Y.-F.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B. Solvent selection and Pt decoration towards enhanced photocatalytic CO2 reduction over CsPbBr3 perovskite single crystals. Sustain. Energy Fuels 2020, 4, 2249–2255. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, N.Y.; Shen, J.Q.; Liao, P.Q.; Chen, X.M.; Zhang, J.P. Hydroxide ligands cooperate with catalytic centers in metal–organic frameworks for efficient photocatalytic CO2 reduction. J. Am. Chem. Soc. 2018, 140, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, Q.; Xu, C.; Zhao, D.; Zhu, Q.; Zhu, Z.; Wang, J.; Liu, C.; Yu, H.; Sun, C.; et al. Current dilemma in photocatalytic CO2 reduction: Real solar fuel production or false positive outcomings? Carbon Neutrality 2022, 1, 10. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, S.; Peter, S.C. Systematic assessment of solvent selection in photocatalytic CO2 reduction. ACS Energy Lett. 2021, 6, 3270–3274. [Google Scholar] [CrossRef]
- Liu, B.J.; Torimoto, T.; Yoneyama, H. Photocatalytic reduction of CO2 using surface-modified CdS photocatalysts in organic solvents. J. Photochem. Photobiol. A Chem. 1998, 113, 93–97. [Google Scholar] [CrossRef]
- Alcasabas, A.; Ellis, P.R.; Malone, I.; Williams, G.; Zalitis, C. A Comparison of Different Approaches to the Conversion of Carbon Dioxide into Useful Products: Part I: CO2 reduction by electrocatalytic, thermocatalytic and biological routes. Johns. Matthey Technol. Rev. 2021, 65, 180–196. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; Jeyalakshmi, V.; Krishnamurthy, K.R.; Viswanathan, B. Photocatalytic reduction of carbon dioxide by water on titania: Role of photophysical and structural properties. Indian J. Chem. 2012, 51A, 411–419. [Google Scholar]
- Wei, Y.; Jiao, J.; Zhao, Z.; Zhong, W.; Li, J.; Liu, J.; Jiang, G.; Duan, A. 3D ordered macroporous TiO2-supported Pt@ CdS core–shell nanoparticles: Design, synthesis and efficient photocatalytic conversion of CO2 with water to methane. J. Mater. Chem. A 2015, 3, 11074–11085. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, P.; Liu, H. Visible-light-driven photoelectrocatalytic degradation of diclofenac by N, S-TiO2/TiO2NTs photoelectrode: Performance and mechanism study. J. Environ. Chem. Eng. 2015, 3, 1713–1719. [Google Scholar] [CrossRef]
- Yahaya, A.H.; Gondal, M.A.; Hameed, A. Selective laser enhanced photocatalytic conversion of CO2 into methanol. Chem. Phys. Lett. 2004, 400, 206–212. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Z.; Zhao, Z.; Liu, Q.; Kou, J.; Chen, X.; Gao, J.; Yan, S.; Zou, Z. High-yield synthesis of ultrathin and uniform Bi2WO6 square nanoplates benefitting from photocatalytic reduction of CO2 into renewable hydrocarbon fuel under visible light. ACS Appl. Mater. Interfaces 2011, 3, 3594–3601. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xiao, T.C.; Edwards, P.P. The use of products from CO2 photoreduction for improvement of hydrogen evolution in water splitting. Int. J. Hydrogen Energy 2011, 36, 6546–6552. [Google Scholar] [CrossRef]
- Razzaq, A.; In, S.-I. TiO2 Based Nanostructures for Photocatalytic CO2 Conversion to Valuable Chemicals. Micromachines 2019, 10, 326. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+ Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Camarillo, R.; Rizaldos, D.; Jiménez, C.; Martínez, F.; Rincón, J. Enhancing the photocatalytic reduction of CO2 with undoped and Cu-doped TiO2 nanofibers synthesized in supercritical medium. J. Supercrit. Fluids 2019, 147, 70–80. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-vacancy modified graphitic carbon nitride: Enhanced CO2 photocatalytic reduction performance and mechanism probing. J. Mater. Chem. A 2019, 7, 1556. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Guo, Y.; Shen, M.; Wang, M.; Li, B.; Shi, J. Multifunctional 2D porous g-C3N4 nanosheets hybridized with 3D hierarchical TiO2 microflowers for selectuive dye adsorption, antibiotic degradation and CO2 reduction. Chem. Eng. J. 2020, 396, 125347. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Wang, M.; Shen, M.; Zhang, L. FeP modified polymeric carbon nitride as a noble-metal free photocatalyst for efficient CO2 reduction. Catal. Comm. 2021, 156, 106326. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, J.; Bai, Y.; Liang, X.; Wang, T.; Wang, C. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping. J. CO2 Util. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Molina-Muriel, M.; Peng, Y.; García, H.; Ribera, A. Increased photocatalytic activity and selectivity towards methane of trimetallic NiTiAl-LDH. J. Alloys Compd. 2022, 897, 163124. [Google Scholar] [CrossRef]
- Wang, X.; Ng, D.; Du, H.; Hornung, C.H.; Polyzos, A.; Seeber, A.; Li, H.; Huo, Y.; Xie, Z. Copper decorated indium oxide rods for photocatalytic CO2 conversion under simulated sun light. J. CO2 Util. 2022, 58, 101909. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Nawawi, M.G.M. Highly stable honeycomb structured 2D/2D vanadium aluminum carbide MAX coupled g-C3N4 composite for stimulating photocatalytic CO2 reduction to CO and CH4 in a monolith photoreactor. J. Alloys Compd. 2022, 927, 166908. [Google Scholar] [CrossRef]
- Movahed, S.K.; Najinasab, A.; Nikbakht, R.; Dabiri, M. Visible light assisted photocatalytic reduction of CO2 to methanol using Fe3O4@NC/Cu2O nanostructure photocatalyst. J. Photochem. Photobiol. A Chem. 2020, 401, 112763. [Google Scholar] [CrossRef]
- Cheng, M.; Bai, S.; Xia, Y.; Zhu, X.; Chen, R.; Liao, Q. Highly efficient photocatalytic conversion of gas phase CO2 by TiO2 nanotube array sensitized with CdS/ZnS quantum dots under visible light. Int. J. Hydrogen Energy 2021, 46, 31634–31646. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wu, S.; Zhang, J. Advances for CO2 Photocatalytic Reduction in Porous Ti-Based Photocatalysts. ACS EST Eng. 2022, 2, 942–956. [Google Scholar] [CrossRef]
- Liu, G.; Hoivik, N.; Wang, K.; Jakobsen, H. Engineering TiO2 nanomaterials for CO2 conversion/solar fuels. Sol. Energy Mater. Sol. Cell. 2012, 105, 53–68. [Google Scholar] [CrossRef]
- Sanz-Marco, A.; Hueso, J.L.; Sebastian, V.; Nielsen, D.; Mossin, S.; Holgado, J.P.; Bueno-Alejo, C.J.; Balas, F.; Santamaria, J. LED-driven controlled deposition of Ni onto TiO2 for visible-light expanded conversion of carbon dioxide into C1–C2 alkanes. Nanoscale Adv. 2021, 3, 3788. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, J.-W.; Zhong, L.; Zhen, W.; Tay, Y.Y.; Li, S.; Wang, Y.-G.; Huang, L.; Xue, C. Synergistic effect of Ru-N4 sites and Cu-N3 sites in carbon nitride for highly selective photocatalytic reduction of CO2 to methane. Appl. Catal. B Environ. 2022, 307, 121154. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Xu, H.; Li, Y.; Wei, Y.; Liu, J.; Zhao, Z. Efficient Z-scheme photocatalysts of ultrathin g-C3N4-wrapped Au/TiO2-nanocrystals for enhanced visible-light-driven conversion of CO2 with H2O. Appl. Catal. B Environ. 2020, 263, 118314. [Google Scholar] [CrossRef]
- Sonowal, K.; Saikia, L. Metal–organic frameworks and their composites for fuel and chemical production via CO2 conversion and water splitting. RSC Adv. 2022, 12, 11686–11707. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, K.; Nandal, N.; Basyach, P.; Kalita, L.; Jain, S.L.; Saikia, L. Photocatalytic reduction of CO2 to methanol using Zr(IV)-based MOF composite with g-C3N4 quantum dots under visible light irradiation. J. CO2 Util. 2022, 57, 101905. [Google Scholar] [CrossRef]
- Zhan, W.; Gao, H.; Yang, Y.; Li, X.; Zhu, Q.-L. Rational Design of Metal–Organic Framework-Based Materials for Photocatalytic CO2 Reduction. Adv. Energy Sustain. Res. 2022, 3, 2200004. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal–Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, Q.; Liu, Y.; Huang, B.; Dai, Y.; Zhang, X.; Qin, X. A bismuth-based metal-organic framework as an efficient visible-light-driven photocatalyst. Chemistry 2015, 21, 2364–2367. [Google Scholar] [CrossRef]
- Xiao, J.D.; Jiang, H.L. Metal-organic frameworks for photocatalysis and photothermal catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, J.; Li, J.; Pahm, T.D.; Zhang, L.; He, J.; Brudvig, G.W.; Deskins, N.A.; Frenkel, A.I.; Li, G. Revealing the structure of single cobalt sites in carbon nitride for photocatalytic CO2 reduction. J. Phys. Chem. C 2022, 126, 8596–8604. [Google Scholar] [CrossRef]
- Xia, P.; Antonietti, M.; Zhu, B.C.; Heil, T.; Yu, J.G.; Cao, S.W. Designing Defective Crystalline Carbon Nitride to Enable Selective CO2 Photoreduction in the Gas Phase. Adv. Funct. Mater. 2019, 29, 1900093. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J. CO2 capture and in situ photocatalytic reduction. Chem. Catal. 2022, 2, 425–438. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Synthesis, characterization and visible light photocatalytic activity of metal based TiO2 monoliths for CO2 reduction. Chem. Eng. J. 2016, 283, 1244–1253. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Li, K.; Chen, S.; Yue, D. Photocatalytic Reactor as a Bridge to Link the Commercialization of Photocatalyst in Water and Air Purification. Catalysts 2022, 12, 724. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Wu, J.C. Photoreduction of CO2 in an optical-fiber photoreactor: Effects of metals addition and catalyst carrier. Appl. Catal. A Gen. 2008, 335, 112–120. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Ray, A.K. Major challenges in the design of a large-scale photocatalytic reactor for water treatment. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng. -Biotechnol. 1999, 22, 253–260. [Google Scholar] [CrossRef]
- Wang, W.; Ku, Y. Photocatalytic degradation of gaseous benzene in air streams by using an optical fiber photoreactor. J. Photochem. Photobiol. A Chemistry. 2003, 159, 47–59. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Kapteijn, F. Monolithic reactors in catalysis: Excellent control. Curr. Opin. Chem. Eng. 2013, 2, 346–353. [Google Scholar] [CrossRef]
- Liou, P.Y.; Chen, S.C.; Wu, J.C.; Liu, D.; Mackintosh, S.; Maroto-Valer, M.; Linforth, R. Photocatalytic CO2 reduction using an internally illuminated monolith photoreactor. Energy Environ. Sci. 2011, 4, 1487–1494. [Google Scholar] [CrossRef]
- He, J.; Janáky, C. Recent advances in solar-driven carbon dioxide conversion: Expectations versus reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef]
- Francis, A.; Shanmuga Priya, S.; Harish Kumar, S.; Sudhakar, K.; Tahir, M. A review on recent developments in solar photoreactors for carbon dioxide conversion to fuels. J. CO2 Util. 2021, 47, 101515. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Kumari, G.; Zhang, X.; Jain, P.K. Opportunities and challenges of solar –energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Lett. 2017, 2, 2058–2070. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603. [Google Scholar] [CrossRef]
- Ješić, D.; Jurković, D.L.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering photocatalytic and photoelectrocatalytic CO2 reduction reactions: Mechanisms, intrinsic kinetics, mass transfer resistances, reactors and multi-scale modelling simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Lu, X.; Luo, X.; Thompson, W.A.; Tan, J.Z.; Maroto-Valer, M.M. Investigation of carbon dioxide photoreduction process in a laboratory-scale photoreactor by computational fluid dynamic and reaction kinetic modeling. Front. Chem. Sci. Eng. 2022, 16, 1149–1163. [Google Scholar] [CrossRef]
- Xiong, Z.; Kuang, C.-C.; Lin, K.-Y.; Lei, Z.; Chen, X.; Gong, B.; Yang, J.; Zhao, Y.; Zhang, J.; Xia, B.; et al. Enhanced CO2 photocatalytic reduction through simultaneously accelerated H2 evolution and CO2 hydrogenation in a twin photoreactor. J. CO2 Util. 2018, 24, 500–508. [Google Scholar] [CrossRef]
- Lin, H.; Luo, S.; Zhang, H.; Ye, J. Toward solar-driven carbon recycling. Joule 2022, 6, 294–314. [Google Scholar] [CrossRef]
- Gui, M.M.; Cathie Lee, W.P.; Putri, L.K.; Kong, X.Y.; Tan, L.-L.; Chai, S.P. Photo-Driven Reduction of Carbon Dioxide: A Sustainable Approach Towards Achieving Carbon Neutrality Goal. Front. Chem. Eng. 2021, 3, 744911. [Google Scholar] [CrossRef]
- Boutin, E.; Patel, M.; Kecsenovity, E.; Suter, S.; Janáky, C.; Haussener, S. Photo-Electrochemical Conversion of CO2 Under Concentrated Sunlight Enables Combination of High Reaction Rate and Efficiency. Adv. Energy Mater. 2022, 12, 2200585. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Duan, J.; Feng, H.; Liu, D.; Li, Q. Solar-Driven Carbon Dioxide Reduction: A Fair Evaluation of Photovoltaic-Biased Photoelectrocatalysis and Photovoltaic-Powered Electrocatalysis. Front. Energy Res. 2022, 10, 956444. [Google Scholar] [CrossRef]






| Ordered Number | Chemical Equation | Electrochemical Redox Potential (V) vs. NHE |
|---|---|---|
| 1 | −2.0 | |
| 2 | −0.41 | |
| 3 | −0.61 | |
| 4 | −0.52 | |
| 5 | −0.48 | |
| 6 | −0.38 | |
| 7 | −0.24 | |
| 8 | −0.166 | |
| 9 | −0.050 | |
| 10 | +0.044 | |
| 11 | +0.478 | |
| 12 | +0.311 | |
| 13 | +0.209 | |
| 14 | +0.145 | |
| 15 | +0.157 |
| Photocatalyst | Pressure and Temperature | Reactants | Light Source | CO | Reactor Type/Catalyst Form | References |
|---|---|---|---|---|---|---|
| TNF (0, 240, 40) | 1 bar He 0.12 bar CO2 | CO2(g), H2O | 450 W Xe arc lamp | 228.3 μmol/g/h | Stainless-steel A glass microfiber filter impregnated with the solid catalyst | [59] |
| Cu-TNF (0.5, 240,60) | 319.62 μmol/g/h | |||||
| GCN | Ambient pressure, temperature kept at 15 °C by cooling water circulation | CO2(g), H2O(g) | 300 W Xe arc lamp | 1.28 μmol/g/h | Air-tight static reactor Photocatalyst deposited on a quartzose plate | [60] |
| GCN-510 | 4.18 μmol/g/h | |||||
| 3D/2D TiO2/p-g-C3N4 (TOCN-100, TOCN-80, TOCN-50, TOCN-20, HCN, HTO) | Reaction temperature kept at 15 °C by cooling water circulation | CO2(g), H2O(g) | Xe lamp with a 420 nm cutoff filter and average light intensity of 210 mW cm−2 | 0.209 0.974 μmol/g/h | Airtight reactor with an optical quartz window at the top | [61] |
| FeP/CN | Ambient pressure, temperature kept at 15 °C by cooling water circulation | CO2(g), H2O(g) | 300 W Xe lamp | 5.19 μmol/g/h | Glass reactor | [62] |
| Photocatalyst | Pressure and Temperature | Reactants | Light Source | Methane | Reactor Type/Catalyst form | References |
|---|---|---|---|---|---|---|
| TNF (0, 200, 80) | 1 bar He 0.12 bar CO2 | CO2(g), H2O | 450 W Xe arc lamp | 62 μmol/g/h | Stainless-steel A glass microfiber filter impregnated with the solid catalyst | [59] |
| Cu-TNF (0.5, 240,60) | 204,6 μmol/g/h | |||||
| TiO2 | Vacuum, 25 °C | CO2 (g), H2O(g) | 300W Xe lamp | 4 μmol/g 5 h | Vacuum reactor system Catalyst sprinkled on a circular quartz plate | [63] |
| 0.1% Mo-TiO2 | 18 μmol/g 5 h | |||||
| 0.3% Mo-TiO2 | 49 μmol/g 5 h | |||||
| 0.5% Mo-TiO2 | 11 μmol/g 5 h | |||||
| NiTiAl-LDH | 0.5 bar CO2 40 °C H2 | CH3CN: H2O:TEOA (3:1:1) Ru(bpy)3Cl2·6H2O | Solar simulator equipped with a Xe arc lamp and an AM 1.5G filter | 148 μmol/g/h | Quartz reactor (55 mL) Dispersed catalyst | [64] |
| In2O3 | 1.0 MPa 40 °C | CO2(g) H2O(l) | 300 W Xe lamp | (CH4 + CO) mmol⋅gcat−1 h−1 1.088 | Stainless-steel autoclave reactor (50 mL) Catalyst suspension | [65] |
| In2O3 with 20% Cu | 1.485 | |||||
| In2O3 with 50% Cu | 1.485 | |||||
| Pristine g-CN | / | CO2, CH3OH | UV (254 nm) 200 W Hg lamp 100 mW cm−2 | 69 μmolg−1 | Fixed bed, stainless chamber integrated with mass flow controllers (MFC) and cooling fans | [66] |
| 5% V2AlC/g-CN | 101 μmolg−1 | |||||
| 10% V2AlC/g-CN | 134 μmolg−1 | |||||
| 10% V2AlC/g-CN | / | CO2, CH3OH | Vis (420 nm) 300 W Xenon reflector lamp 100 mW cm−2 | 53.6 μmolg−1 |
| Photocatalyst | Pressure and Temperature | Reactants | Light Source | Methanol | Reactor Type | Reference |
|---|---|---|---|---|---|---|
| Fe3O4@N-C/Cu2O (12 wt% of Cu2O) | ambient conditions (temperature controlled by a thermostatic water bath at 25 ± 3 °C) | CO2(g), H2O (1.5 mL), DMF (13.5 mL) | 5 W Xenon HID lamp | 440 μmol gcat−1 3 h | Reaction vessel Dispersed catalyst | [67] |
| Fe3O4@N-C/Cu2O (12 wt% of Cu2O) | ambient conditions | CO2, H2O, acetonitrile | 5 W Xenon HID lamp | 410 μmol gcat−1 3 h | Reaction vessel | [67] |
| CeO2 pristine ceria sample | at room temperature | H2O, NaHCO3, 0.35 M HCl | visible light/ 300 W xenon light | 0.5 μmol g−1 in 1 h | 150 mL quartz reactor | [17] |
| UT-CeOx ultrasound treated | 1.8 μmol g−1 in 1 h | |||||
| UT-CeOx | 7.2 μmol g−1 in 4 h | |||||
| TNTA 10 SILAR | 60 °C | CO2(g) (flow rate = 20.5 mL/min) H2O(g) | visible light xenon lamp 50 mW/cm2 | 70.80 nmol/(cm2·h) 6 h 193.63 nmol/(cm2·h) 6 h | Optofluidic planar microreactor (high 0.04 cm, reaction area 2.5cm x 2cm) | [68] |
| CdS/ZnS-TNTA 10 SILAR | 193.63 nmol/(cm2·h) 6 h | |||||
| visible light xenon lamp 110 mW/cm2 | 255.49 nmol/(cm2·h) 6 h | |||||
| 3.74% N-In2O3 | 25 °C, 1.01 bar | CO2(g), H2O(l), 10 vol.% TEOA | Xenon lamp (300 W) | 394 μmol gcat−1 h−1 6 h | Gas circulation- evacuation reactor Dispersed photocatalyst | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratovčić, A.; Tomašić, V. Design and Development of Photocatalytic Systems for Reduction of CO2 into Valuable Chemicals and Fuels. Processes 2023, 11, 1433. https://doi.org/10.3390/pr11051433
Bratovčić A, Tomašić V. Design and Development of Photocatalytic Systems for Reduction of CO2 into Valuable Chemicals and Fuels. Processes. 2023; 11(5):1433. https://doi.org/10.3390/pr11051433
Chicago/Turabian StyleBratovčić, Amra, and Vesna Tomašić. 2023. "Design and Development of Photocatalytic Systems for Reduction of CO2 into Valuable Chemicals and Fuels" Processes 11, no. 5: 1433. https://doi.org/10.3390/pr11051433
APA StyleBratovčić, A., & Tomašić, V. (2023). Design and Development of Photocatalytic Systems for Reduction of CO2 into Valuable Chemicals and Fuels. Processes, 11(5), 1433. https://doi.org/10.3390/pr11051433