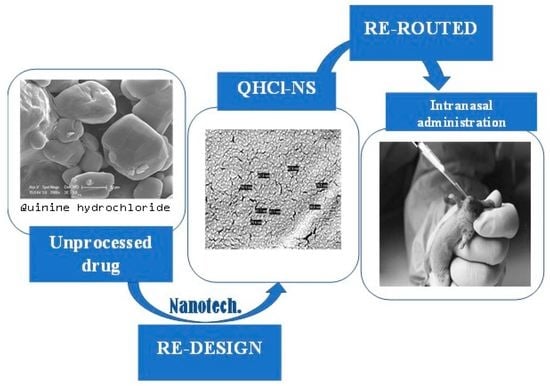
Quinine: Redesigned and Rerouted
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of QHCl Solubility in Solid and Liquid Lipids
2.2.2. Formulation of SRMS Lipid Matrices
2.2.3. Preparation of QHCl-NS
2.2.4. Characterization of QHCl-NS
Determination of Particle Sizes, Polydispersity Indices, and Zeta (ζ) Potentials of NS
Determination of the Morphology of QHCl-NS
- (1)
- Transmission Electron Microscopy (TEM) of QHCl
- (2)
- Cryo-Scanning Electron Microscopy (cryo-SEM) and Field Emission SEM (FESEM) of NS
Fourier Transform Infrared (FTIR) Spectroscopic Analysis
Thermal Analysis
Powder X-ray Diffractometry (XRD)
Time-Dependent pH Stability Studies of NS
Osmolality Determination
2.2.5. Solubility Analysis of QHCl in Simulated Nasal Fluid and Alcoholic Buffer
2.2.6. In Vitro Release Studies of QHCl-NS
2.2.7. Ex Vivo Permeation Studies of QHCl-NS
2.2.8. In Vivo Pharmacodynamic Studies
2.2.9. Histopathological Studies
2.2.10. Data and Statistical Analysis
3. Results and Discussion
3.1. Solubility of QHCl in Solid Lipids and Liquid Lipids
3.2. Mean Particle Size and Particle Distribution Indices Analyses of QHCl-NS
3.2.1. Stability Studies of QHCl-NS
3.2.2. Surface Charge (Zeta (ζ) Potential) of NS
3.3. Time-Dependent pH Stability Studies and Osmolality of QHCl-NS
3.4. Morphology of QHCl-NS
3.5. FTIR Spectroscopic Analysis
3.6. Crystalline State of QHCl-NS
3.7. Powder X-ray Diffractometry of NS
3.8. In Vitro Release Studies of QHCl-NS
3.8.1. In Vitro Release of QHCl-NS
3.8.2. Evaluation of Drug Release Mechanism and Kinetics of QHCl-NS
3.9. Ex Vivo Permeation Analysis of QHC1-NS
3.10. In Vivo Antimalarial Studies of QHCl-NS
3.11. Histopathological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; Alessandro, U.D. Quinine, an Old Anti-Malarial Drug in a Modern World: Role in the Treatment of Malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guideline for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015. [CrossRef]
- Fairhurst, R.M.; Wellems, T.E. Malaria (Plasmodium Species). In Mandell Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3070–3090. [Google Scholar]
- Fairhurst, R.M.; Dondrop, A.M. Artemisinin-Resistant Plasmodium Falciparum Malaria. Microbiol. Spectr. 2016, 4, 4-3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzondu, S.; Echezona, A.; Nwagwu, C.; Onugwu, A.; Ugorji, L.; Agbo, C.; Kenechukwu, F.; Ogbonna, J.; Akpa, P.; Nnamani, P.; et al. Combating Antimalarial Drug Resistance: Recent Advances and Future Perspectives; IntechOpen: London, UK, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. GLS-1200 Topical Nasal Spray to Prevent SARS-CoV-2 Infection (COVID-19). NCT04408183. Available online: https://clinicaltrials.gov/ct2/show/NCT04408183 (accessed on 31 August 2022).
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinine. In Meyler’s Side Effects of Drugs; Aronson, J.K. (Ed.) Elsevier: Oxford, UK, 2016; pp. 27–35. [Google Scholar] [CrossRef]
- Agbo, C.P.; Ugwuanyi, T.C.; Ugwuoke, W.I.; McConville, C.; Attama, A.A.; Ofokansi, K.C. Intranasal Artesunate-Loaded Nanostructured Lipid Carriers: A Convenient Alternative to Parenteral Formulations for the Treatment of Severe and Cerebral Malaria. J. Control. Release 2021, 334, 224–236. [Google Scholar] [CrossRef]
- Gupta, Y.; Jain, A.; Jain, S.K. Transferrin-conjugated Solid Lipid Nanoparticles for Enhanced Delivery of Quinine Dihydrochloride to the Brain. J. Pharm. Pharmacol. 2007, 59, 935–940. [Google Scholar] [CrossRef]
- Marijon, A.; Bonnot, G.; Fourier, A.; Bringer, C.; Lavoignat, A.; Gagnieu, M.-C.; Bienvenu, A.-L.; Picot, S. Efficacy of Intranasal Administration of Artesunate in Experimental Cerebral Malaria. Malar. J. 2014, 13, 501. [Google Scholar] [CrossRef] [Green Version]
- Torrino, E.; De Marco, I.; Reverchon, E. Organic Nanoparticles Recovery in Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2010, 55, 300–306. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Frankline, K.C.; Ugwu, C.E.; Musiliu, A.O.; Agboke, A.A.; Agbo, P.; Ossai, E.C.; Ofomata, A.C.; Youngson, D.C.; Omeje, C.E.; et al. Development and Evaluation of Artemether-Loaded Microspheres Delivery System for Oral Application in Malaria Treatment. Trop. J. Nat. Prod. Res. 2021, 5, 2030–2036. [Google Scholar]
- Wang, C.; Yan, T.; Yan, T.; Wang, Z. Fabrication of Hesperetin/Hydroxypropyl-B-Cyclodextrin Complex Nanoparticles for Enhancement of Bioactivity Using Supercritical Antisolvent Technology. J. Mol. Struct. 2023, 1279, 134947. [Google Scholar] [CrossRef]
- Haas, S.E.; Bettoni, C.C.; de Oliveira, L.K.; Guterres, S.S.; Costa, T.D. Nanoencapsulation Increases Quinine Antimalarial Efficacy against Plasmodium berghei In Vivo. Int. J. Antimicrob. Agents 2009, 34, 156–161. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of Quality by Design Approach for Intranasal Delivery of Rivastigmine Loaded Solid Lipid Nanoparticles: Effect on Formulation and Characterization Parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Agbo, C.; Umeyor, C.; Kenechukwu, F.; Ogbonna, J.; Chime, S.; Lovelyn, C.; Agubata, O.; Ofokansi, K.; Attama, A. Formulation Design, in vitro Characterizations and Anti-Malarial Investigations of Artemether and Lumefantrine-Entrapped Solid Lipid Microparticles. Drug Dev. Ind. Pharm. 2016, 42, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Sood, S.; Gowthamarajan, K. Optimization of Artemether-Loaded NLC for Intranasal Delivery Using Central Composite Design. Drug Deliv. 2015, 22, 940–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured Lipid Carrier System for Topical Delivery of Terbinafine Hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Tichota, D.M.; Silva, A.C. Design, Characterization, and Clinical Evaluation of Argan Oil Nanostructured Lipid Carriers to Improve Skin Hydration. Int. J. Nanomed. 2014, 20, 3855–3864. [Google Scholar]
- Pretorius, E. Influence of Acceleration Voltage on Scanning Electron Microscopy of Human Blood Platelets. Microsc. Res. Technol. 2010, 73, 225–228. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified Chitosan Thermosensitive Hydrogel Enables Sustained and Efficient Anti-Tumor Therapy via Intratumoral Injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef]
- Mallappa, P.; Prabirkumar, S.; Panchakshari, A. Taste Masked Quinine Sulphate Loaded Solid Lipid Nanoparticles for Flexible Pediatric Dosing. Indian J. Pharm. Educ. Res. 2014, 48, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Momoh, M.A.; Franklin, K.C.; Agbo, C.P.; Ugwu, C.E.; Adedokun, M.O.; Anthony, O.C.; Chidozie, O.E.; Okorie, A.N. Microemulsion-Based Approach for Oral Delivery of Insulin: Formulation Design and Characterization. Heliyon 2020, 6, e03650. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Agbo, C.P.; Nwagwu, C.S.; Uzondu, E.; Echezona, A.C.; Dike, J.; Ogbonna, N.; Akpa, P.A.; Momoh, M.A.; Nnamani, P.O.; et al. Development of Lipid-Based Microsuspensions for Improved Ophthalmic Delivery of Gentamicin Sulphate. Ther. Deliv. 2021, 12, 671–683. [Google Scholar] [CrossRef]
- FDA. Dissolution Methods Database; FDA: Washington, DC, USA, 2019.
- Ümİt, G.; Melİke, Ü.; Gülgün, Y.; Ecem Fatma, K.; Aydoğmuş, Z. Formulation and Characterization of Solid Lipid Nanoparticles, Nanostructured Lipid Carriers and Nanoemulsion of Lornoxicam for Transdermal Delivery. Acta Pharm. 2015, 65, 771–791. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative Study of Transmembrane Diffusion and Permeation of Ibuprofen across Synthetic Membranes Using Franz Diffusion Cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Clemmer, L.; Martins, Y.C.; Zanini, G.M.; Frangos, J.A.; Carvalho, L.J.M. Artemether and Artesunate Show the Highest Efficacies in Rescuing Mice with Late-Stage Cerebral Malaria and Rapidly Decrease Leukocyte Accumulation in the Brain. Antimicrob. Agents Chemother. 2011, 55, 1383–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.G.; Grau, G.E.; Janse, C.; Kazura, J.W.; Milner, D.; Barnwell, J.W.; Turner, G.; Langhorne, J.; on behalf of the participants of the Hinxton Retreat meeting on “Animal Models for Research on Severe Malaria”. The Role of Animal Models for Research on Severe Malaria. PLoS Pathog. 2012, 8, e1002401. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S. Routes of Administration. In The Laboratory Mouse (Handbook of Experimental Animals)—USP; Elsevier: Tsukuba, Japan, 2004; pp. 527–541. [Google Scholar]
- Simmons, M.L.; Brick, J.O. The Laboratory Mouse; Hollaender, A., Ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1970. [Google Scholar]
- Shen, X.; Lagergård, T.; Yang, Y.; Lindblad, M.; Fredriksson, M.; Holmgren, J.A.N.; Mmun, I.N.I. Group B Streptococcus Capsular Polysaccharide-Cholera Toxin B Subunit Conjugate Vaccines Prepared by Different Methods for Intranasal Immunization. Infect. Immun. 2001, 69, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Lagergård, T.; Yang, Y.; Lindblad, M.; Fredriksson, M.; Holmgren, J.A.N. Systemic and Mucosal Immune Responses in Mice after Mucosal Immunization with Group B Streptococcus Type III Capsular Polysaccharide-Cholera Toxin B Subunit Conjugate Vaccine. Infect. Immun. 2000, 68, 5749–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoori, Y.; Mohkam, M.; Heidari, R.; Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Golkar, N.; Gholami, A. Development and In vivo Characterization of Probiotic Lysate- Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing. Biomed Res. Int. 2020, 2020, 8868618. [Google Scholar] [CrossRef]
- Gratieri, T.; Martins, G.; Melani, E.; Hugo, V.; Freitas, O.; De Fonseca, R.; Lopez, V. A Poloxamer/Chitosan in Situ Forming Gel with Prolonged Retention Time for Ocular Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Khames, A. Investigation of the Effect of Solubility Increase at the Main Absorption Site on Bioavailability of BCS Class II Drug (Risperidone) Using Liquisolid Technique. Drug Deliv. 2017, 24, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Gattefossé. Transcutol® P For Efficient Drug Solubilization and Skin Penetration; Gattefossé: Saint-Priesr Cedex, France, 2020; pp. 1–24. Available online: https://www.gattefosse.com/pharmaceuticals-products/transcutol-p (accessed on 29 April 2023).
- Salimi, M.; Fouladi, A. Effect of the Various Penetration Enhancers on the In vitro Skin Permeation of Meloxicam through Whole Rat Skin. Eur. J. Bio. Pharm. Sci 2015, 2, 1282–1291. [Google Scholar]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A Review of the Nonclinical Safety of Transcutol Ò, a Highly Purified Form of Diethylene Glycol Monoethyl Ether (DEGEE) Used as a Pharmaceutical Excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef]
- Zirak, M.B.; Pezeshki, A. Effect of Surfactant Concentration on the Particle Size, Stability and Potential Zeta of Beta Carotene Nano Lipid Carrier. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 924–932. [Google Scholar]
- Softisan. Available online: www.warnergraham.com/images/SoftisanHardFatsProdIn.pdf (accessed on 10 February 2022).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glyceryl-behenate (accessed on 10 February 2022).
- Gycerol Dibehenate. European Pharmacopeia; Conseil de l’Europe: Strasbourg, French, 2007; pp. 2110–2111. [Google Scholar]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Development and In Vivo Evaluation of an Innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” Formulation with Sustained Release and Enhanced Oral Bioavailability for Potential Hypertension Treatment in Pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Radomska-Soukharev, A.; Muller, R.H. Chemical Stability of Lipid Excipients in SLN-Production of Test Formulations, Characterization and Short-Term Stability. Pharmazie 2006, 61, 425–430. [Google Scholar]
- Tan, S.F.; Masoumi, H.R.F.; Karjiban, R.A.; Stanslas, J.; Kirby, B.P.; Basri, M.; Basri, H.B. Ultrasonic Emulsification of Parenteral Valproic Acid-Loaded Nanoemulsion with Response Surface Methodology and Evaluation of Its Stability. Ultrason. Sonochem. 2016, 29, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Shridharan, P.; Sivakumar, M. Impact of Process Parameters in the Generation of Novel Aspirin Nanoemulsions—Comparative Studies between Ultrasound Cavitation and Microfluidizer. Ultrason. Sonochem. 2013, 20, 485–497. [Google Scholar] [CrossRef]
- Aoki, M.; Ring, T.A.; Haggerty, J.S. Analysis and Modeling of the Ultrasonic Dispersion Technique. Adv. Ceram. Mater. 1987, 2, 209–212. [Google Scholar] [CrossRef]
- Özdemir, S.; Çelik, B.; Üner, M. Properties and Therapeutic Potential of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Promising Colloidal Drug Delivery Systems. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 451–499. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and Poloxamines in Nanoparticle Engineering and Experimental Medicine. Trends Biotechnol. 2000, 18, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of Surfactants on the Formation and Characterization of a New Type of Collodial Drug Delivery System: Nanostructured Lipid Carriers. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Appasaheb, P.S. A Review on Intranasal Drug Delivery System. J. Adv. Pharm. Edu. Res. 2013, 3, 333–346. [Google Scholar]
- Thorat, S. Formulation and Product Development of Nasal Spray: An Overview. Sch. J. Appl. Med. Sci. 2016, 4, 2976–2985. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of Lipid Nanoparticles by Differential Scanning Calorimetry, X-ray and Neutron Scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Alonso, M.J.; Torres, D. Design and Characterization of a New Drug Nanocarrier Made from Solid–Liquid Lipid Mixtures. J. Colloid Interface Sci. 2005, 285, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.Q.; Zeng, S. Preparation and Characterization of Stearic Acid Nanostructured Lipid Carriers by Solvent Diffusion Method in an Aqueous System. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Jia, L.-J.; Zhang, D.-R.; Li, Z.-Y.; Feng, F.-F.; Wang, Y.-C.; Dai, W.-T.; Duan, C.-X.; Zhang, Q. Preparation and Characterization of Silybin-Loaded Nanostructured Lipid Carriers. Drug Deliv. 2010, 17, 11–18. [Google Scholar] [CrossRef]
- Boyer, R.F. Transitions and Relaxations in Amorphous and Semicrystalline Organic Polymers and Copolymers. Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 1977; pp. 745–839. [Google Scholar]
- Boutonnet-Fagegaltier, N.; Menegotto, J.; Lamure, A.; Duplaa, H.; Caron, A.; Lacabanne, C.; Bauer, M. Molecular Mobility Study of Amorphous and Crystalline Phases of a Pharmaceutical Product by Thermally Stimulated Current Spectrometry. J. Pharm. Sci. 2002, 91, 1548–1560. [Google Scholar] [CrossRef]
- Brandl, F.; Kastner, F.; Gschwind, R.M.; Blunk, T.; Teßmar, J.; Göpferich, A. Release Kinetics. J. Control. Release 2009, 142, 221–228. [Google Scholar] [CrossRef]
- Marx, D.; Williams, G.; Birkhoff, M. Intranasal Drug Administration—An Attractive Delivery Route for Some Drugs. In Drug Discovery and Development; IntechOpen: London, UK, 2015; pp. 1–23. [Google Scholar] [CrossRef] [Green Version]
- Basir, R.; Rahiman, S.F.; Hasballah, K.; Chong, W.; Talib, H.; Yam, M.; Jabbarzare, M.; Tie, T.; Othman, F.; Moklas, M.; et al. Plasmodium Berghei ANKA Infection in ICR Mice as a Model of Cerebral Malaria. Iran. J. Parasitol. 2012, 7, 62–74. [Google Scholar] [PubMed]











| QHCl-NS | Lipid Type | Concentration (%) | Cup-Horn Sonication Time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QHCl | Solid Lipid | MCT | THP | T80 | P188 | Sorbitol | |||
| Q1 | S154 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 30 |
| Q3 | S154 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 90 |
| Q9 | S154 | 1.5 | 3 | 1.5 | 1.5 | 2 | 1 | 5 | 60 |
| Q10 | S154 | 1.5 | 3 | 1.5 | 1.5 | 5 | 1 | 5 | 60 |
| Q13 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 60 |
| Q14 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 60 |
| Q15 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 60 |
| Q5 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 2 | 1 | 5 | 30 |
| Q6 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 5 | 1 | 5 | 30 |
| Q7 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 2 | 1 | 5 | 90 |
| Q8 | CHD 5 | 1.5 | 3 | 1.5 | 1.5 | 5 | 1 | 5 | 90 |
| Q2 | C888 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 30 |
| Q4 | C888 | 1.5 | 3 | 1.5 | 1.5 | 3 | 1 | 5 | 90 |
| Q11 | C888 | 1.5 | 3 | 1.5 | 1.5 | 2 | 1 | 5 | 60 |
| Q12 | C888 | 1.5 | 3 | 1.5 | 1.5 | 5 | 1 | 5 | 60 |
| Formulation/API | Group | Treatment | Dosing | Route |
|---|---|---|---|---|
| QHCl | G1 | Q9 | 20 mg/kg at 0 h, 10 mg/kg every 12 h for 4 days | IN |
| G2 | Plain QHCl solution | |||
| G3 | Q9 | Oral | ||
| Placebo | G4 | Blank NS | 20 mg/kg at 0 h, 10 mg/kg every 12 h for 4 days | IN |
| G5 | Oral |
| Solid Lipids | Stearic Acid | Softisan® 154 | Compritol® HD 5 ATO | Compritol® 888 ATO |
|---|---|---|---|---|
| QHCl Solubility | + | ++ | +++ | ++ |
| Liquid Lipids | Glyceryl monooleate | Transcutol® HP | ||
| QHCl Solubility | +++ | +++ | ++++ |
| QHCl-NS | 24 h | 30 Days | 90 Days | ||||
|---|---|---|---|---|---|---|---|
| Particle Size (nm) | PDI | Zeta Potential | Particle Size (nm) | PDI | Particle Size (nm) | PDI | |
| Q1 | 194.76 ± 4.495 | 0.441 ± 0.006 | 6.72 ± 0.259 | 199.27 ± 0.4225 | 0.433 ± 0.011 | 192.1 ± 2.371 | 0.427 ± 0.013 |
| Q3 | 118.4 ± 0.7216 | 0.426 ± 0.017 | 3.24 ± 0.304 | 143.4 ± 0.6202 | 0.406 ± 0.2646 | 177.0 ± 3.704 | 0.237 ± 0.016 |
| Q9 | 117.5 ± 1.53 | 0.282 ± 0.004 | 6.95 ± 0.416 | 112.2 ± 1.715 | 0.279 ± 0.004 | 113.7 ± 0.7550 | 0.279 ± 0.007 |
| Q10 | 80.9 ± 1.57 | 0.465 ± 0.015 | 4.74 ± 0.371 | 68.15 ± 0.8786 | 0.456 ± 0.003 | 60.85 ± 0.5901 | 0.442 ± 0.013 |
| Q13 | 92.9 ± 2.765 | 0.564 ± 0.032 | 4.81 ± 0.067 | 97.51 ± 0.767 | 0.431 ± 0.029 | 90.58 ± 0.747 | 0.315 ± 0.003 |
| Q14 | 109.4 ± 0.814 | 0.599 ± 0.011 | 4.92 ± 0.096 | 115.2 ± 0.7092 | 0.443 ± 0.005 | 130.2 ± 0.4173 | 0.211 ± 0.011 |
| Q15 | 120.5 ± 4.828 | 0.574 ± 0.027 | 4.28 ± 0.180 | 97.67 ± 1.640 | 0.425 ± 0.015 | 104.2 ± 0.7024 | 0.280 ± 0.003 |
| Q5 | 83.52 ± 0.676 | 0.467 ± 0.008 | 6.19 ± 0.396 | 86.92 ± 0.999 | 0.319 ± 0.020 | 92.82 ± 0.5046 | 0.255 ± 0.010 |
| Q6 | 150 ± 1.595 | 0.475 ± 0.007 | 5.36 ± 0.106 | 120.5 ± 0.808 | 0.371 ± 0.006 | 130.8 ± 0.625 | 0.268 ± 0.005 |
| Q7 | 68.6 ± 0.861 | 0.491 ± 0.003 | 5.93 ± 0.18 | 79.9 ± 2.87 | 0.261 ± 0.010 | 87.31 ± 1.160 | 0.225 ± 0.005 |
| Q8 | 90.36 ± 0.520 | 0.445 ± 0.003 | 4.36 ± 0.076 | 90.50 ± 2.859 | 0.307 ± 0.028 | 97.51 ± 0.197 | 0.307 ± 0.028 |
| Q2 | 300.8 ± 10.11 | 0.603 ± 0.029 | 4.23 ± 0.294 | 206 ± 27.97 | 0.596 ± 0.146 | 186.9 ± 16.12 | 0.557 ± 0.073 |
| Q4 | 121.5 ± 39.62 | 0.397 ± 0.132 | 2.31 ± 0.061 | 121.5 ± 39.62 | 0.397 ± 0.132 | 143.3 ± 1.739 | 0.441 ± 0.012 |
| Q11 | 119.4 ± 0.945 | 0.511 ± 0.008 | 0.738 ± 0.138 | 109.1 ± 1.649 | 0.488 ± 0.006 | 113.2 ± 4.192 | 0.475 ± 0.017 |
| Q12 | 118.6 ± 1.093 | 0.492 ± 0.022 | 2.16 ± 0.659 | 103.5 ± 1.580 | 0.434 ± 0.007 | 111.8 ± 4.925 | 0.318 ± 0.037 |
| Batches | Osmolality (mOsmol/kg) | Flux (µg/cm2min) | Permeation Coefficient (cm/s) | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | ||||
| Q7 | 422.3 ± 12.3 | - | - | 0.7807 | 0.9161 | 0.9939 | 0.9756 | 0.5055 |
| Q9 | 492.7 ± 17.9 | 320.710 | 2.18 × 10−2 | 0.7466 | 0.8885 | 0.9298 | 0.9993 | 0.8869 |
| Q12 | 517.0 ± 21.7 | - | - | 0.8603 | 0.9611 | 0.9710 | 1 | 0.7730 |
| Plain Solution of QHCl | - | 56.973 | 3.87 × 10−4 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbo, C.P.; Ugwuanyi, T.C.; Eze, O.C.; Onugwu, A.L.; Echezona, A.C.; Nwagwu, C.S.; Uzondu, S.W.; Ogbonna, J.D.; Ugorji, L.O.; Nnamani, P.O.; et al. Quinine: Redesigned and Rerouted. Processes 2023, 11, 1811. https://doi.org/10.3390/pr11061811
Agbo CP, Ugwuanyi TC, Eze OC, Onugwu AL, Echezona AC, Nwagwu CS, Uzondu SW, Ogbonna JD, Ugorji LO, Nnamani PO, et al. Quinine: Redesigned and Rerouted. Processes. 2023; 11(6):1811. https://doi.org/10.3390/pr11061811
Chicago/Turabian StyleAgbo, Chinazom Precious, Timothy Chukwuebuka Ugwuanyi, Osita Christopher Eze, Adaeze Linda Onugwu, Adaeze Chidiebere Echezona, Chinekwu Sherridan Nwagwu, Samuel Wisdom Uzondu, John Dike Ogbonna, Lydia Onyinyechi Ugorji, Petra Obioma Nnamani, and et al. 2023. "Quinine: Redesigned and Rerouted" Processes 11, no. 6: 1811. https://doi.org/10.3390/pr11061811
APA StyleAgbo, C. P., Ugwuanyi, T. C., Eze, O. C., Onugwu, A. L., Echezona, A. C., Nwagwu, C. S., Uzondu, S. W., Ogbonna, J. D., Ugorji, L. O., Nnamani, P. O., Akpa, P. A., Reginald-Opara, J. N., Ogbodo, J. O., McConville, C., Attama, A. A., Momoh, M. A., & Ofokansi, K. C. (2023). Quinine: Redesigned and Rerouted. Processes, 11(6), 1811. https://doi.org/10.3390/pr11061811