Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prickly Pear Fruits and Seeds
2.2. Chemicals and Reagents
2.3. Determination of Moisture Content of PPSO
2.4. PPSO Isolation Using Soxhlet Extraction
2.5. Determination of the Volatile Compounds of Prickly Pear Seed Oil (PPSO)
2.6. GC/MS Instrumentation and Conditions of Analysis
2.7. Identification of the Volatile Compounds of PPSO
2.8. Determination of Free Fatty Acids (FAs) of PPSO
2.9. Determination of In Vitro Antioxidant Activity (IVAA) and Total Phenolic Content (TPC) of PPSO
2.10. Statistical Analysis
3. Results and Discussion
3.1. Contribution of Seeds to the Total Fruit Mass
3.2. Moisture Content of Prickly Pear Fruits and Seeds
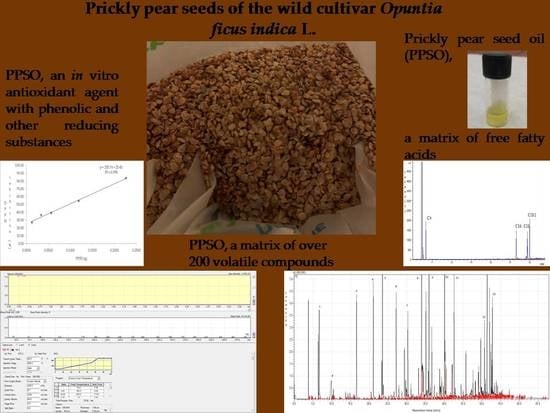
3.3. Volatile Compounds of PPSO
3.4. Oil Yield and Fatty Acid Profile of PPSO
3.5. IVAA and TPC of PPSO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil composition and characterization of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.D.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Vanessa YelinaMontañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Tomás Filardo-Kerstupp, T. Antioxidant and antimicrobial properties of cactus pear (Opuntia) seed oils. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Ramírez-Moreno, E.; Sánchez-Mata, M.C.; Carvalho, A.M.; Ferreira, I.C. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle F.A.C. Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 2012, 46, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Regalado-Rentería, E.; Aguirre-Rivera, J.R.; González-Chávez, M.M.; Sánchez-Sánchez, R.; Martínez-Gutiérrez, F.; Juárez-Flores, B.I. Assessment of extraction methods and biological value of seed oil from eight variants of prickly pear fruit (Opuntia spp.). Waste Biomass Valoriz. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.-J.; Zu, Y.-G.; Tong, M.-H.; Wu, N.; Liu, X.-L.; Zhang, S. Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem. 2009, 114, 334–339. [Google Scholar]
- Matthäus, B.; Özcan, M.M. Habitat effects on yield, fatty acid composition and tocopherol contents of prickly pear (Opuntia ficus-indica L.) seed oils. Sci. Hortic. 2011, 131, 95–98. [Google Scholar] [CrossRef]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.M.; Senatore, F. Essential oils composition of two Sicilian cultivars of Opuntia ficus-indica (L.) Mill. (Cactaceae) fruits (prickly pear). Nat. Prod. Res. 2013, 27, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Mobraten, K.; Haug, T.M.; Kleiveland, C.R.; Lea, T. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Heal. Dis. 2013, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Sindic, M.; Aziz, M.; Bnouham, M. Evaluation of antidiabetic properties of cactus pear seed oil in rats. Pharm. Boil. 2014, 52, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, M.; Hugo, A. Oil content and fatty acid composition of cactus pear seed compared with cotton and grape seed. J. Food Biochem. 2010, 34, 93–100. [Google Scholar] [CrossRef]
- Gatzias, I.; Karabagias, I.; Kontakos, S.; Kontominas, M.; Badeka, A. Characterization and differentiation of sheep’s milk from Greek breeds based on physicochemical parameters, fatty acid composition and volatile profile. J. Sci. Food Agric. 2018, 98, 3935–3942. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Riganakos, K.A. Characterization of prickly pear juice by means of shelf life, sensory notes, physicochemical parameters and bio-functional properties. J. Food Sci. Technol. 2019, 56, 3646–3659. [Google Scholar] [CrossRef] [PubMed]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, F.; Hasib, A.; Ouatmane, A.; Elbatal, H.; Jaouad, A. Physicochemical characteristics of Moroccan prickly pear juice (Opuntia ficus indica L.). Int. J.Emerg. Technol. Adv. Eng. 2014, 4, 300–306. [Google Scholar]
- Martin, A.; Armbruster, U.; Atia, H. Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur. J. Lipid Sci. Technol. 2011, 114, 10–23. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2016, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Vermeulen, N.; Czerny, M.; Gänzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
- Gassenmeier, K.; Schieberle, P. Formation of the intense flavor compound trans-4,5-epoxy-(E)-2-decenal in thermally treated fats. J. Am. Oil Chem. Soc. 1994, 71, 1315–1319. [Google Scholar] [CrossRef]
- Konopka, U.C.; Grosch, W. Potent odorants causing the warmed-over flavour in boiled beef. Eur. Food Res. Technol. 1991, 193, 123–125. [Google Scholar] [CrossRef]
- Rota, V.; Schieberle, P. Changes in key odorants of sheep meat induced by cooking. In Food Lipids; Chapter 6; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2005; Volume 920, pp. 73–83. [Google Scholar]
- Buckingham, J. Dictionary of Organic Compounds 1, 6th ed.; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Oomah, B.D.; Razafindrainibe, M.; Drover, J.C. Headspace volatile components of Canadian grown low-tannin faba bean (Vicia faba L.) genotypes. J. Sci. Food Agric. 2014, 94, 473–481. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Louppis, A.; Badeka, A.; Kontominas, M.G.; Papastephanou, C. Valorization of prickly pear juice geographical origin based on mineral and volatile compound contents using LDA. Foods 2019, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- El Mannoubi, I.; Barrek, S.; Skanji, T.; Casabianca, H.; Zarrouk, H. Characterization of Opuntia ficus indica seed oil from Tu;nisia. Chem. Nat. Compd. 2009, 45, 616–620. [Google Scholar] [CrossRef]
- Ennouri, M.; Evelyne, B.; Laurence, M.; Hamadi, A. Fatty acid composition and rheological behavior of prickly pear seed oils. Food Chem. 2005, 93, 431–437. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.-T.; Hassanien, M.F.R. Oil cactus pear (Opuntia ficus-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bruno, M.; Balzano, M.; Giardinieri, A.; Pacetti, D.; Frega, N.G.; Vincenzo, S.; Leporini, M.; Tundis, R. Comparative chemical composition and bioactivity of Opuntia ficus-indica Sanguigna and Surfarina seed oils obtained by traditional and ultrasound-assisted extraction procedures. Eur. J. Lipid Sci. Technol. 2019, 121, 1800283. [Google Scholar] [CrossRef] [Green Version]
- Ghazi, Z.; Ramdani, M.; Fauconnier, M.L.; El Mahi, B.; Cheikh, R. Fatty acids sterols and vitamin E composition of seed oil of Opuntia ficus indica and Opuntia dillenii from Morocco. J. Mater. Environ. Sci. 2013, 4, 967–972. [Google Scholar]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef]
- Chaalal, M.; Touati, N.; Louaileche, H. Extraction of phenolic compounds and in vitro antioxidant capacity of prickly pear seeds. Acta Bot. Gallica 2012, 159, 467–475. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Badeka, A.V. Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste? Processes 2020, 8, 132. https://doi.org/10.3390/pr8020132
Karabagias VK, Karabagias IK, Gatzias I, Badeka AV. Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste? Processes. 2020; 8(2):132. https://doi.org/10.3390/pr8020132
Chicago/Turabian StyleKarabagias, Vassilios K., Ioannis K. Karabagias, Ilias Gatzias, and Anastasia V. Badeka. 2020. "Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste?" Processes 8, no. 2: 132. https://doi.org/10.3390/pr8020132
APA StyleKarabagias, V. K., Karabagias, I. K., Gatzias, I., & Badeka, A. V. (2020). Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste? Processes, 8(2), 132. https://doi.org/10.3390/pr8020132