Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Moisture and Oil Content
2.3. Preliminary Experiments
2.4. Experimental Design
2.5. Heating Pretreatment of Bulk Pumpkin Seeds
2.6. Compression Tests and Calculated Responses
2.7. Spectrophotometric Analysis of Oil Samples
2.8. Determination of Chemical Properties of Oils Samples
2.9. Statistical Evaluation of Experimental Data
3. Results
3.1. Preliminary Test of Pumpkin Seeds under LaboratoryTemperature
3.2. Effect of Pretreatment on Force-Deformation Curves of Pumpkin Seeds
3.3. Spectral Curves of Pumpkin Seeds Oil at Pretreatment Temperatures
3.4. Chemical Properties of Oil Samples
3.5. Box–Behnken Design of the Factors Combination
3.6. Determined Regression Models of the Responses
3.7. Optimum Processing Factors for Oil Extraction
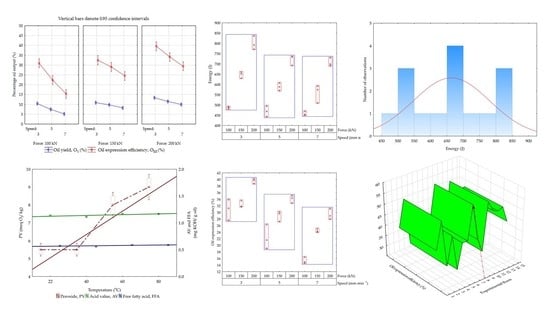
3.8. Percentage Error, Pareto Chart, and Box–Cox Effects on the Responses
3.9. Fitted Response Surface Plots Verses Processing Factors
3.10. Determination of Oil Point Force at Optimum Factors
3.11. Compression and Relaxation Curves at Optimum Factors
3.12. Description of the Supplementary Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozberk, Z.A.; Ergonul, P.G. Chapter 18–Cold pressed pumpkin seed oil. In Green Technology, Bioactive Compounds, Functionality and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 219–229. [Google Scholar]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 2019, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgin, U.; Korkmaz, H. A green separation process for recovery of healthy oil from pumpkin seed. J. Supercrit. Fluids 2011, 58, 239–248. [Google Scholar] [CrossRef]
- El-Adaway, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef]
- Can-Cauich, C.; Sauri-Duch, E.; Moo-Huchin, V.M.; Betancur-Ancona, D. Effect of extraction and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico. Food Chem. 2019, 285, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Amador, R.M.; Fregapane, G.; Salvador, M.D. Composition and properties of virgin pistachio oils and their by-products from different cultivars. Food Chem. 2018, 240, 123–130. [Google Scholar] [CrossRef]
- Amin, M.Z.; Rity, T.I.; Uddin, M.R.; Rahman, M.M.; Uddin, M.J. A comparative assessment of anti-inflammatory, anti-oxidant and anti-bacterial activities of hybrid and indigenous varieties of pumpkin seed oil. Biocatal. Agric. Biotechnol. 2020, 28, 1–7. [Google Scholar] [CrossRef]
- Xie, J.M. Induced polarization effect of pumpkin protein on B16 cell. FJMU 2004, 38, 394–395. [Google Scholar]
- Xia, H.C.; Li, F.; Zhang, Z.C. Purification and characterization of Moschatin, a novel type I ribosome-inactivating protein from the mature seeds of pumpkin (Cucurbita moschata), and preparation of its immunotoxin against human melanoma cells. Cell Res. 2003, 13, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Ilio, C.D. Escherichia coli in Europe an overview. Int. J. Environ. Res. Public Health. 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Yoon, J.; Hovde, C.J. A brief overview of Escherichia coli 0157:H7 and its plasmid 0157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todar, K. Pathogenic E. coli. Online Textbook of Bacteriology; Department of Bacteriology, University of Wisconsin-Madison: Madison, WI, USA, 2007; pp. 1–30. [Google Scholar]
- Nishimura, M.; Ohkawara, T.; Sato, H.; Takeda, H.; Nishihira, J. Pumpkin seed oil extracted from Cucurbita maxima improves urinary disorder in human overactive Bladder. J. Tradit. Complement. Med. 2014, 4, 72–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Giovanni, M.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; D’Amico, L.; Piro, G.; Mita, G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO2 extracted pumpkin (Cucurbita moschata Duch.) oil. Food Chem. 2014, 148, 314–320. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Z.G.; Gai, Q.Y.; Li, X.J.; Wei, F.Y.; Fu, Y.J.; Ma, W. Microwaveassisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, L.; Mathieu, H.; Mhemdi, H.; Vorobiev, E. Characterization of oilseeds mechanical expression in an instrumented pilot screw press. Ind. Crops Prod. 2018, 121, 106–113. [Google Scholar] [CrossRef]
- Karaj, S.; Muller, J. Optimizing mechanical oil extraction of Jatropha curcas L. seeds with respect to press capacity, oil recovery and energy efficiency. Ind. Crops Prod. 2011, 34, 1010–1016. [Google Scholar]
- Mpagalile, J.J.; Hanna, M.A.; Weber, R. Seed oil extraction using a solar powered screw press. Ind. Crops Prod. 2007, 25, 101–107. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Ojeda-Amador, R.M.; Gomez-Alonso, S.; Salvador, M.D.; Fregapane, G.; Hamdi, S. Cucurbita maxima pumpkin seed oil: From the chemical properties to the different extracting techniques. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Roohinejad, S.; Greiner, R.; Vorobiev, E. Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: An overview. Food Res. Int. 2016, 85, 59–66. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Teong, L.K.; JitKang, L. Comparative exergy analyses of Jatropha curcas oil extraction methods: Solvent and mechanical extraction processes. Energy Convers. Manag. 2012, 55, 164–171. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Wiesenborn, D.P.; Tostenson, K.; Kangas, N. Energy analysis I the screw pressing of whole and dehulled flaxseed. J. Food Eng. 2005, 66, 193–202. [Google Scholar] [CrossRef]
- Wiesenborn, D.; Doddapaneni, R.; Tostenson, K.; Kangas, N. Cooking indices to predict screw-press performance for crambe seed. J. Am. Oil Chem. Soc. 2001, 78, 467–471. [Google Scholar] [CrossRef]
- Sivakumaran, K.; Goodrum, J. Laboratory oilseed processing by a small screw press. J. Am. Oil Chem. Soc. 1988, 65, 932–935. [Google Scholar] [CrossRef]
- Olayanju, T.; Akinoso, R.; Oresanya, M. Effect of wormshaft speed, moisture content and variety on oil recovery from expelled beniseed. Agric. Eng. Int. CIGR J. 2006, 43, 181–183. [Google Scholar]
- Olayanju, T. Effect of wormshaft speed and moisture content on oil and cake qualities of expelled sesame seed. Trop. Sci. 2003, 43, 181–183. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.L.; Wiesenborn, D.P.; Tostenson, K.; Kangas, N. Screw pressing of whole and dehulled flaxseed for organic oil. J. Am. Oil Chem. Soc. 2003, 80, 1039–1045. [Google Scholar] [CrossRef]
- Ward, J. Pre-pressing of oil from rapeseed and sunflower. J. Am. Oil Chem. Soc. 1994, 61, 1358–1361. [Google Scholar] [CrossRef]
- Sigalingging, R.; Herak, D.; Kabutey, A.; Cestmir, M.; Divisova, M. Tangent curve function description of mechanical behaviour of bulk oilseeds: A review. Sci. Agric. Bohem. 2014, 45, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Sigalingging, R.; Herak, D.; Kabutey, A.; Dajbych, O.; Hrabe, P.; Cestmir, M. Application of a tangent curve mathematical model for analysis of the mechanical behaviour of sunflower bulk seeds. Int. Agrophys. 2015, 29, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Divisova, M.; Herak, D.; Kabutey, A.; Sigalingging, R.; Svatonova, T. Deformation curve characteristics of rapeseeds and sunflower seeds under compression loading. Sci. Agric. Bohem. 2014, 45, 180–186. [Google Scholar]
- Munson-Mcgee, S.H. D-optimal experimental designs for uniaxial expression. J. Food Process Eng. 2014, 37, 248–256. [Google Scholar] [CrossRef]
- Jaya, S.; Durance, T.D. Stress relaxation behaviour of microwave-vacuum-dried alginate gels. J. Texture Stud. 2008, 39, 183–197. [Google Scholar] [CrossRef]
- Kandar, M.I.M.; Akil, H.M. Application of design of experiment (DoE) for parameters optimization in compression moulding for flax reinforced biocomposites. Procedia Chem. 2016, 19, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Salamatinia, B.; Mootabadi, H.; Hashemizadeh, I.; Abdullah, A.Z. Intensification of biodiesel production from vegetable oils using ultrasonic-assisted process: Optimization and kinetic. Chem. Eng. Process. 2013, 73, 135–143. [Google Scholar] [CrossRef]
- Box, G.E.P.; Draper, N.R. Empirical Model-Building and Response Surfaces; John Willey and Sons: New York, NY, USA, 1987. [Google Scholar]
- ISI. Indian Standard Methods for Analysis of Oilseeds; IS:3579; ISI: New Delhi, India, 1966. [Google Scholar]
- Huang, S.; Hu, Y.; Li, F.; Jin, W.; Godara, V.; Wu, B. Optimization of mechanical oil extraction process from Camellia oleifera seeds regarding oil yield and energy consumption. J. Food Process Eng. 2019, 42, 1–11. [Google Scholar] [CrossRef]
- Blahovec, J. Agromatereials Study Guide; Czech University of Life Sciences Prague: Prague, Czech Republic, 2008. [Google Scholar]
- Niu, L.; Li, J.; Chen, M.S.; Xu, Z.F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind. Crops Prod. 2014, 56, 187–190. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Chanioti, S.; Constantina, T. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Ocholi, O.; Menkiti, M.; Auta, M.; Ezemagu, I. Optimization of the operating parameters for the extractive synthesis of biolubricant from sesame seed oil via response surface methodology. Egypt. J. Pet. 2018, 27, 265–275. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modeling and optimization of biosorption process. Bioresour. Technol. 2014, 60, 150–160. [Google Scholar] [CrossRef]
- Hernandez-Santos, B.; Rodriguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-Garcia, R.; Juarez-Barrientos, J.M.; Chavez-Zamudio, R.; Martinez-Sanchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Deli, S.; Farah Masturah, M.; Tajul Aris, Y.; Wan Nadiah, W.A. The effects of physical parameters of the screw press oil expeller on oil yield from Nigella sativa L. seeds. Int. Food Res. J. 2011, 18, 1367–1373. [Google Scholar]
- Orozco, F.D.A.; Sousa, A.C.; Araujo, M.C.U.; Domini, C.E. A new flow UV-Vis kinetics spectrophotometric method based on photodegradative reaction for determining the oxidative stability of biodiesel. Fuel 2020, 26, 116–197. [Google Scholar]
- Gurkan, A.K.G.; Kabutey, A.; Selvi, K.C.; Hrabe, P.; Herak, D.; Frankova, A. Investigation of heating and freezing pretreatments of mechanical, chemical and spectral properties of bulk sunflower seeds and oil. Processes 2020, 8, 411. [Google Scholar]
- Chatepa, L.E.C.; Uluko, H.; Masamba, K. Comparison of oil quality extracted from selected conventional and non conventional sources of vegetable oil from Malawi. Afr. J. Biotechnol. 2019, 18, 171–180. [Google Scholar]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physiochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Statsoft Inc. STATISTICA for Windows; Statsoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Rodrigues, J.; Miranda, I.; Gominho, J.; Vasconcelos, M.; Barradas, G.; Pereira, H.; Bianchi-de-Aguiar, F.; Ferreira-Dias, S. Modeling and optimization of laboratory-scale conditioning of Jatropha curcas L. Seeds for oil expression. Ind. Crops Prod. 2016, 83, 614–619. [Google Scholar] [CrossRef]
- Lv, H.; Liu, H.; Tan, Y.; Meng, A.; Assogba, O.C.; Xiao, S. An extended search method for identifying optimal parameters of the generalized Maxwell model. Contr. Build. Mater. 2021, 266, 1–13. [Google Scholar] [CrossRef]
- Salimi, A.; Abbassi-Sourki, F.; Karrabi, M.; Ghoreishy, M.H.R. Investigation on viscoelastic behaviour of virgin EPDM/reclaimed rubber blends using generalized Maxwell model (GMM). Polym. Test. 2021, 93, 106989. [Google Scholar] [CrossRef]
- Zhang, Z.; Bader, Y.M.K.; Lucian, A.L.; Yang, J. Improved stress relaxation resistance of composites films by soy protein polymer. Compos. Commun. 2021, 24, 100644. [Google Scholar] [CrossRef]
- Malomuzh, N.P.; Shakun, K.S. Maxwell relaxation time for argon and water. J. Mol. Liq. 2019, 293, 111413. [Google Scholar] [CrossRef]
- Herak, D.; Kabutey, A.; Choteborsky, R.; Petru, M.; Sigalingging, R. Mathematical models describing the relaxation behaviour of Jatropha curcas L. bulk seeds under axial compression. Biosyst. Eng. 2015, 131, 77–83. [Google Scholar] [CrossRef]
- Nobile, M.A.D.; Chillo, S.; Mentana, A.; Baiano, A. Use of the generalized Maxwell model for describing the stress relaxation behaviour of solid-like foods. J. Food Eng. 2007, 78, 978–983. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterization of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Sirisomboon, P.; Kitchaiya, P. Physical properties of Jatropha curcas L. kernels after heat treatments. Biosyst. Eng. 2009, 102, 244–250. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; De Haan, A.B. A consolidation based extruder model to explore GAME process configurations. J. Food Eng. 2009, 90, 238–245. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; De Haan, A.B. Hydraulic pressing of oilseeds; experimental determination and modelling of yield and pressing rates. J. Food Eng. 2008, 89, 8–16. [Google Scholar] [CrossRef]
- Beerens, P. Screw Pressing of Jatropha Seeds for Fueling Purposes in Less Developed Countries. Master’s Thesis, Department of Sustainable Energy Technology, Eindhoven University of Technology, Eindhoven, The Netherlands, 2007; p. 87. [Google Scholar]
- Pritchard, P.J. Mathcad: A Tool for Engineering Problem Solving; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Petru, M.; Novak, O.; Herak, D.; Simanjuntak, S. Finite element method model of the mechanical behaviour of Jatropha curcas L. seeds under compression loading. Biosyst. Eng. 2012, 114, 412–421. [Google Scholar] [CrossRef]
- Jolivet, P.; Deruyffelaere, C.; Boulard, C.; Quinsac, A.; Savoire, R.; Nesi, N.; Chardot, T. Deciphering the structural organization of the oil bodies in the Brassica napus seed as a mean to improve the oil extraction yield. Ind. Crops Prod. 2013, 44, 549–557. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.; Calderon-Dominguez, G.; Chanona-Perez, J.; Farrera-Rebollo, R.R.; Mendez-Mendez, J.V.; Diaz-Ramirez, M. Chia (Salvia hispanica L.) seed mucilage release characterisation. A microstructural and image analysis study. Ind. Crops Prod. 2013, 51, 453–462. [Google Scholar] [CrossRef]
- Mayerhofer, T.G.; Popp, J. Beer’s law derived from electromagnetic theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, Y.; Guan, L.; Wu, S.; Li, D. A turbidity compensation method for COD measurements by UV-vis spectroscopy. Optik 2019, 186, 129–136. [Google Scholar] [CrossRef]
- Gobrecht, A.; Bendoula, R.; Roger, J.M.; Bellon-Maurel, V. Combining linear polarization spectroscopy and the representative layer theory to measure the Beer-Lambert law absorbance of highly scattering materials. Anal. Chim. Acta 2015, 853, 486–494. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Pushkarev, V.E.; Tomilova, L.G. A mathematical analysis of deviations from linearity of Beer’s law. Chem. Phys. Lett. 2018, 706, 520–524. [Google Scholar] [CrossRef]
- Wang, L.; Ayaz, H.; Izzetoglu, M.; Onaral, B. Evaluation of light detector surface area for functional Near Infrared Spectroscopy. Comput. Biol. Med. 2017, 89, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Mantele, W.; Deniz, E. UV-VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef]
- Maikala, R. Modified Beer’s law—Historical perspectives and relevance in near-infrared monitoring of optical properties of human tissue. Int. J. Ind. Ergon. 2010, 40, 125–134. [Google Scholar] [CrossRef]
- Kumar, K.A.; Viswanathan, K. Study of UV Transmission through a Few Edible Oils and Chicken Oil. J. Spectrosc. 2013, 2013, 540417. [Google Scholar]
- Okene, E.O.; Evbuomwan, B.O. Solvent extraction and characteristics of oil from coconut seeds using alternative solvents. Int. J. Eng. Sci. Technol. 2014, 2, 135–138. [Google Scholar]
- Lozada, M.I.O.; Maldonade, I.R.; Rodrigues, D.B.; Santos, D.S.; Sanchez, B.A.O.; De Souza, P.E.N.; Longo, J.P.; Amaro, G.B.; De Oliveira, L.D.L. Physicochemical characterization and nano-emulsification of three species of pumpkin seed oils with focus on their physical stability. Food Chem. 2021, 343, 1–13. [Google Scholar]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterization of pumpkin seed oil. Ind. Crops Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Evangelista, R.L.; Isbell, T.A.; Cermak, S.C. Extraction of pennycress (Thlaspi arvense L.). Ind. Crops Prod. 2012, 37, 76–81. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Gu, H.; Yang, L. An approach for extraction of kernel oil from Pinus pumila using homogenate-circulating ultrasound in combination with an aqueous enzymatic process and evaluation of its antioxidant activity. J. Chromatogr. 2016, 147, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Seymen, M.; Uslu, N.; Turkmen, O.; Juhaimi, F.A.; Ozcan, M.M. Chemical compositions and mineral contents of some hull-less pumpkin seed and oils. J. Am. Oil Chem. Soc. 2016, 93, 1095–1099. [Google Scholar] [CrossRef]
- Younis, Y.M.H.; Ghirmay, S.; Al-Shihry, S.S. African Cucurbita pepo L.: Properties of seed and variability in fatty acid composition of seed oil. Phytochemistry 2000, 54, 71–75. [Google Scholar] [CrossRef]
- Ekop, I.E.; Simonyan, K.J.; Onwuka, U.N. Effects of processing factors and conditions on the cracking efficiency of Tympanotonus fuscatus and Pachymelania aurita periwinkles: Response surface approach. J. Agric. Food Res. 2021, 3, 100094. [Google Scholar] [CrossRef]
- Almasi, S.; Ghobadian, B.; Najafi, G.H.; Yusaf, T.; Soufi, M.D.; Hoseini, S.S. Optimization of an ultrasound-assisted biodiesel production process from one genotype of rapeseed (TERI (OE) R-983) as a novel feedstock using response surface methodology. Energies 2019, 12, 2656. [Google Scholar] [CrossRef] [Green Version]
- Jalili, F.; Jafari, S.M.; Emam-Djomeh, Z.; Malekjani, N. Optimization of ultrasound-assisted extraction of oil from canola seeds with the use of response surface methodology. Food. Anal. Methods 2018, 11, 598–612. [Google Scholar] [CrossRef]
- Gohi, B.F.C.A.; Du, J.; Zeng, H.-Y.; Cao, X.-J.; Zou, K.M. Microwave pretreatment and enzymolysis optimization of the lotus seed protein. Bioengineering 2019, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; Taitai, A. Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef] [Green Version]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the extraction process of sesame seed’s oil using response surface method on the industrial scale. Ind. Crops Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- Akangbe, O.L.; Blahovec, J.; Adamovsky, R.; Linda, M.; Hromasova, M. A device to measure wall friction during uniaxial compression of biomaterials. In Proceedings of the 7th TAE, Prague, Czech Republic, 17–20 September 2019; pp. 14–19. [Google Scholar]
- Domokos, G.; Sipos, A.; Szabo, T. Pebbles, Shapes, and Equilibria. Math. Geosci. 2010, 42, 29–47. [Google Scholar] [CrossRef]
- Tien, Y.M.; Wu, P.L.; Huang, W.H.; Kuo, M.F.; Chu, C.A. Wall friction measurement and compaction characteristics of bentonite powders. Powder Technol. 2007, 173, 140–151. [Google Scholar] [CrossRef]
- Illenberger, W. Pebble shape (and size!). J. Sediment Res. 1991, 61, 756. [Google Scholar]
- Sneed, E.; Folk, R.L. Pebbles in the lower Colorado River, Texas, a study in particle morphogenesis. J. Geol. 1958, 66, 114–150. [Google Scholar] [CrossRef]
- Zingg, T. Beitrag zur Schotteranalyse. Schweiz Mineral Petroger Mitt. 1935, 15, 39–40. [Google Scholar]
- Yin, W.; Washington, M.; Ma, X.; Yang, X.; Lu, A.; Shi, R.; Zhao, R.; Wang, X. Consumer acceptability and sensory profiling of sesame oils obtained from different processes. Oil Gas Sci. Technol. 2020, 3, 39–48. [Google Scholar] [CrossRef]
- Fernandes, G.D.; Ellis, A.C.; Gambaro, A.; Barrera-Arellano, D. Sensory evaluation of high-quality virgin olive oil: Panel analysis versus consumer perception. Curr. Opin. Food Sci. 2018, 21, 66–71. [Google Scholar] [CrossRef]
- Amelio, M. The official method for olive oil sensory evaluation: An expository revision of certain sections of the method and a viable means for confirming the attribute intensities. Trends Food Sci. Technol. 2015, 47, 64–68. [Google Scholar] [CrossRef]
- Lauri, I.; Pagano, B.; Malmendal, A.; Sacchi, R.; Novellino, E.; Randazzo, A. Application of ‘’magnetic tongue’’ to the sensory evaluation of extra virgin olive oil. Food Chem. 2013, 140, 692–699. [Google Scholar] [CrossRef] [PubMed]
















| Speed (mm/min) | Force (kN) | N | (mm) | |||
|---|---|---|---|---|---|---|
| 3 | 100 | 3 | 44.58 ± 2.27 | 10.41 ± 1.05 | 30.96 ± 3.12 | 487.77 ± 4.94 |
| 3 | 150 | 3 | 48.91 ± 0.33 | 10.91 ± 0.34 | 32.45 ± 1.00 | 647.94 ± 15.11 |
| 3 | 200 | 3 | 48.76 ± 0.64 | 13.31 ± 0.23 | 39.59 ± 0.69 | 800.70 ± 32.48 |
| 5 | 100 | 3 | 45.96 ± 0.90 | 7.52 ± 1.22 | 22.36 ± 3.64 | 469.99 ± 25.32 |
| 5 | 150 | 3 | 47.33 ± 2.32 | 9.76 ± 0.51 | 29.03 ± 1.52 | 589.17 ± 17.92 |
| 5 | 200 | 3 | 47.61 ± 1.42 | 11.47 ± 0.37 | 34.11 ± 1.10 | 721.57 ± 24.15 |
| 7 | 100 | 3 | 47.48 ± 0.43 | 5.17 ± 0.32 | 15.36 ± 0.94 | 458.45 ± 9.50 |
| 7 | 150 | 3 | 47.01 ± 1.61 | 8.23 ± 0.17 | 24.49 ± 0.50 | 559.53 ± 42.69 |
| 7 | 200 | 3 | 48.57 ± 0.78 | 9.88 ± 0.52 | 29.39 ± 1.56 | 707.46 ± 19.95 |
| Effect | Test | Value | F-Value | Effect df | Error df | p-Value |
|---|---|---|---|---|---|---|
| Intercept | Wilks | 0.00 | 14,925.31 | 3.00 | 16.00 | <0.05 * |
| Speed (mm/min) | Wilks | 0.08 | 13.87 | 6.00 | 32.00 | <0.05 * |
| Force (kN) | Wilks | 0.02 | 29.53 | 6.00 | 32.00 | <0.05 * |
| Speed × Force | Wilks | 0.36 | 1.65 | 12.00 | 42.62 | >0.05 ns |
| Temperature °C | Peroxide Value (PV), (meq O2/kg) | Acid Value (AV), (mg KOH/g oil) | Free Fatty Acid (FFA), mg KOH/g |
|---|---|---|---|
| 22 | 5.5 ± 0.707 | 1.136 ± 0.019 | 0.571 ± 0.009 |
| 40 | 5.5 ± 0.707 | 1.112 ± 0.016 | 0.559 ± 0.008 |
| 60 | 8 ± 1.414 | 1.167 ± 0.016 | 0.587 ± 0.008 |
| 80 | 9 ± 1.414 | 1.166 ± 0.016 | 0.586 ± 0.008 |
| Dependent Variables | R | R2 | F | P |
|---|---|---|---|---|
| PV | 0.845 | 0.714 | 14.964 | <0.05 |
| AV | 0.641 | 0.409 | 4.157 | >0.05 |
| FFA | 0.641 | 0.411 | 4.188 | >0.05 |
| Dependent Variable | Model | Standard Error | T-Value | P |
|---|---|---|---|---|
| Intercept | 3.594 | 0.958 | 3.750 | <0.05 |
| Temperature | 0.067 | 0.017 | 3.868 | <0.05 |
| Run | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 100 (–1) | 3 (–1) | 60 (0) | 77.44 | 66.54 | 10.90 | 44.32 |
| 2 | 200 (1) | 3 (–1) | 60 (0) | 77.44 | 63.28 | 14.16 | 48.05 |
| 3 | 100 (–1) | 7 (1) | 60 (0) | 77.44 | 68.99 | 8.45 | 43.87 |
| 4 | 200 (1) | 7 (1) | 60 (0) | 77.44 | 65.56 | 11.88 | 47.88 |
| 5 | 100 (–1) | 5 (0) | 40 (–1) | 77.44 | 69.98 | 7.46 | 45.00 |
| 6 | 200 (1) | 5 (0) | 40 (–1) | 77.44 | 66.93 | 10.51 | 47.27 |
| 7 | 100 (–1) | 5 (0) | 80 (1) | 77.44 | 66.94 | 10.50 | 46.66 |
| 8 | 200 (1) | 5 (0) | 80 (1) | 77.44 | 62.84 | 14.60 | 47.60 |
| 9 | 150 (0) | 3 (–1) | 40 (−1) | 77.44 | 67.28 | 10.16 | 45.18 |
| 10 | 150 (0) | 7 (1) | 40 (–1) | 77.44 | 69.02 | 8.42 | 42.99 |
| 11 | 150 (0) | 3 (–1) | 80 (1) | 77.44 | 63.18 | 14.26 | 45.15 |
| 12 | 150 (0) | 7 (1) | 80 (1) | 77.44 | 64.49 | 12.95 | 45.52 |
| 13 | 150 (0) | 5 (0) | 60 (0) | 77.44 | 66.77 | 10.67 | 44.73 |
| 14 | 150 (0) | 5 (0) | 60 (0) | 77.44 | 66.52 | 10.92 | 46.60 |
| 15 | 150 (0) | 5 (0) | 60 (0) | 77.44 | 66.54 | 10.90 | 45.66 |
| Run | (°C) | |||||
|---|---|---|---|---|---|---|
| 1 | 100 (–1) | 3 (–1) | 60 (0) | 14,08 | 41,85 | 537.36 |
| 2 | 200 (1) | 3 (–1) | 60 (0) | 18,29 | 54,37 | 834.26 |
| 3 | 100 (–1) | 7 (1) | 60 (0) | 10,91 | 32,45 | 492.24 |
| 4 | 200 (1) | 7 (1) | 60 (0) | 15,34 | 45,62 | 804.13 |
| 5 | 100 (–1) | 5 (0) | 40 (–1) | 9,63 | 28,64 | 502.56 |
| 6 | 200 (1) | 5 (0) | 40 (–1) | 13,57 | 40,36 | 767.76 |
| 7 | 100 (–1) | 5 (0) | 80 (1) | 13,56 | 40,32 | 514.91 |
| 8 | 200 (1) | 5 (0) | 80 (1) | 18,85 | 56,06 | 832.03 |
| 9 | 150 (0) | 3 (–1) | 40 (–1) | 13,12 | 39,01 | 701.97 |
| 10 | 150 (0) | 7 (1) | 40 (–1) | 10,87 | 32,33 | 599.31 |
| 11 | 150 (0) | 3 (–1) | 80 (1) | 18,41 | 54,76 | 696.76 |
| 12 | 150 (0) | 7 (1) | 80 (1) | 16,72 | 49,73 | 645.96 |
| 13 | 150 (0) | 5 (0) | 60 (0) | 16,36 | 48,65 | 662.55 |
| 14 | 150 (0) | 5 (0) | 60 (0) | 16,68 | 49,61 | 675.98 |
| 15 | 150 (0) | 5 (0) | 60 (0) | 14,08 | 41,85 | 671.73 |
| Effect | Standard Error | Sum of Squares, SS | DF | Mean Square, MS | F-Value | |
|---|---|---|---|---|---|---|
| Intercept | 15.71 | 0.59 | 110.5 | 9 | 12.27 | 11.93 * |
| 2.23 | 0.36 | 39.93 | 1 | 39.93 | 38.80 * | |
| –0.97 | 0.53 | 3.44 | 1 | 3.44 | 3.35 ns | |
| –1.26 | 0.36 | 12.62 | 1 | 12.62 | 12.26 * | |
| –0.09 | 0.53 | 0.03 | 1 | 0.03 | 0.03 ns | |
| 2.54 | 0.36 | 51.77 | 1 | 51.77 | 50.31 * | |
| –0.84 | 0.53 | 2.58 | 1 | 2.58 | 2.51 ns | |
| 0.05 | 0.51 | 0.01 | 1 | 0.01 | 0.01 ns | |
| 0.34 | 0.51 | 0.46 | 1 | 0.46 | 0.45 ns | |
| 0.14 | 0.51 | 0.08 | 1 | 0.08 | 0.07 ns | |
| Residual | 5.15 | 5 | 1.03 | |||
| Lack of Fit | 1.10 | 3 | 0.37 | 0.18 ns | ||
| Total SS | 115.62 | 14 |
| Effect | Model b | Standard Error | Sum of Squares, SS | DF | Mean Square, MS | F-Value |
|---|---|---|---|---|---|---|
| Intercept | 46.70 | 1.74 | 976.8 | 9 | 108.53 | 11.93 * |
| 6.64 | 1.07 | 353.02 | 1 | 353.02 | 38.80 * | |
| –2.87 | 1.57 | 30.45 | 1 | 30.45 | 3.35 ns | |
| –3.73 | 1.07 | 111.55 | 1 | 111.55 | 12.26 * | |
| –0.26 | 1.57 | 0.25 | 1 | 0.25 | 0.03 ns | |
| 7.56 | 1.07 | 457.76 | 1 | 457.76 | 50.31 * | |
| –2.49 | 1.57 | 22.85 | 1 | 22.85 | 2.51 ns | |
| 0.16 | 1.51 | 0.11 | 1 | 0.11 | 0.01 ns | |
| 1.01 | 1.51 | 4.06 | 1 | 4.06 | 0.45 ns | |
| 0.41 | 1.51 | 0.68 | 1 | 0.68 | 0.07 ns | |
| Residual | 45.45 | 5 | 9.09 | |||
| Lack of Fit | 3.25 | 3 | 3.25 | 0.18 ns | ||
| Total SS | 1022.28 | 14 |
| Effect. | Standard Error | Sum of Squares, SS | DF | Mean Square, MS | F-Value | |
|---|---|---|---|---|---|---|
| Intercept | 670.09 | 8.57 | 187,554.5 | 9 | 20,839.39 | 94.64 * |
| 148.89 | 5.25 | 177,341.09 | 1 | 177,341.09 | 3764.24 * | |
| –4.89 | 7.72 | 88.21 | 1 | 88.21 | 1.87 ns | |
| –28.59 | 5.25 | 6538.17 | 1 | 6538.17 | 138.78 | |
| 1.80 | 7.72 | 11.94 | 1 | 11.94 | 0.25 ns | |
| 14.76 | 5.25 | 1742.37 | 1 | 1742.37 | 36.98* | |
| –10.89 | 7.72 | 437.48 | 1 | 437.48 | 9.29 ns | |
| 3.75 | 7.42 | 56.15 | 1 | 56.15 | 1.19 ns | |
| 12.98 | 7.42 | 673.84 | 1 | 673.84 | 14.30 ns | |
| 12.97 | 7.42 | 672.50 | 1 | 672.50 | 14.27 ns | |
| Residual | 1101.02 | 5 | 220.20 | |||
| Lack of Fit | 1006.8 | 3 | 335.6 | 7.12 ns | ||
| Total SS | 188,655.5 | 14 |
| Responses | Optimum Values (Profiles for Predicted) | Predicted Values (Regression Model) | Validated Values (Experimental) |
|---|---|---|---|
| (%) | 19.02 | 20.48 | 19.06 * 19.78 a 20.76 * 21.70 b |
| (%) | 56.56 | 60.90 | 56.66 * 58.83 a 61.74 * 64.53 b |
| (J) | 837.33 | 848.04 | 826.10 *a 841.97 *b |
| Responses | Values | PE (%) |
|---|---|---|
| 21.70 20.48 | 5.96 | |
| 64.53 60.90 | 5.96 | |
| (J)
| 841.97 848.04 918.15 | 0.72 8.30 |
| Variable | ||||
|---|---|---|---|---|
| = 5 (0) | 42.25 (46.96 **) | 49.87 | 6.99 | 331.88 |
| * = 4 (–0.5) | 42.48 (46.96 **) | 57.32 | 8.68 | 360.77 |
| Samples N | A (kN) | B (mm–1) | N (–) | Fratio (–) | Fcritical (–) | P value (–) | R2 (–) |
|---|---|---|---|---|---|---|---|
| 1 | 5.848 | 0.031 | 2 | 1.15·10–14 | 3.865 | 0.991 | 0.999 |
| 2 | 5.404 | 0.03 | 2 | 1.07·10–3 | 3.847 | 0.974 | 1 |
| 3 | 5.029 | 0.03 | 2 | 8.712·10–4 | 3.847 | 0.976 | 1 |
| Mean ± SD | 5.427 ± 0.410 | 0.03 ± 0.001 | 2 | 6.471·10–4 ± 0.001 | 3.853 ± 0.010 | 0.980 ± 0.009 | 0.999 ± 0.001 |
| CV (%) | 7.55 | 1.90 |
| Samples N | (MPa) | (MPa) | (MPa) | (MPa · s−1) | (MPa · s−1) |
|---|---|---|---|---|---|
| 1 | 39.597 | 9.357 | 38.658 | 421.245 | 990.532 |
| 2 | 33.474 | 8.445 | 44.196 | 356.102 | 881.34 |
| 3 | 37.476 | 9.823 | 39.293 | 346.998 | 893.019 |
| Mean ± SD | 36.849 ± 3.109 | 9.208 ± 0.701 | 40.712 ± 3.031 | 374.781 ± 40.495 | 921.630 ± 59.966 |
| Samples N | (–) | (–) | (–) | R2 (–) |
|---|---|---|---|---|
| 1 | 0.035 | 3.855 | 0.853 | 0.996 |
| 2 | 0.032 | 3.856 | 0.857 | 0.994 |
| 3 | 0.045 | 3.856 | 0.833 | 0.995 |
| Mean ± SD | 0.037 ± 0.007 | 3.856 ± 0.001 | 0.847 ± 0.013 | 0.995 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabutey, A.; Mizera, Č.; Dajbych, O.; Hrabě, P.; Herák, D.; Demirel, C. Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading. Processes 2021, 9, 540. https://doi.org/10.3390/pr9030540
Kabutey A, Mizera Č, Dajbych O, Hrabě P, Herák D, Demirel C. Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading. Processes. 2021; 9(3):540. https://doi.org/10.3390/pr9030540
Chicago/Turabian StyleKabutey, Abraham, Čestmír Mizera, Oldřich Dajbych, Petr Hrabě, David Herák, and Cimen Demirel. 2021. "Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading" Processes 9, no. 3: 540. https://doi.org/10.3390/pr9030540
APA StyleKabutey, A., Mizera, Č., Dajbych, O., Hrabě, P., Herák, D., & Demirel, C. (2021). Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading. Processes, 9(3), 540. https://doi.org/10.3390/pr9030540