Gold(III) Complexation in the Presence of the Macropolyhedral Hydridoborate Cluster [B20H18]2−
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Materials and Methods
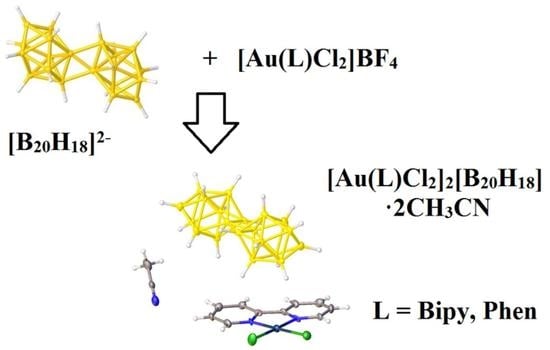
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Muetterties, E.L.; Knoth, W.H. Polyhedral Boranes; Dekker: New York, NY, USA, 1968. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier: Oxford, UK, 1997. [Google Scholar]
- Hosmane, N.S. Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Boustani, I. Boron Clusters, Molecular Modelling and Synthesis of Nanomaterials; Springer Series in Materials Science; Springer: Cham, Switzerland, 2020; Volume 290, pp. 113–254. [Google Scholar] [CrossRef]
- Duttwyler, S. Recent advances in B–H functionalization of icosahedral carboranes and boranes by transition metal catalysis. Pure Appl. Chem. 2018, 90, 733–744. [Google Scholar] [CrossRef]
- Beckett, M.A.; Brellochs, B.; Chizhevsky, I.T.; Damhus, T.; Hellwich, K.-H.; Kennedy, J.D.; Laitinen, R.; Powell, W.H.; Rabinovich, D.; Viñas, C.; et al. Nomenclature for boranes and related species (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 92, 355–381. [Google Scholar] [CrossRef] [Green Version]
- King, R.B. Three-Dimensional Aromaticity in Polyhedral Boranes and Related Molecules. Chem. Rev. 2001, 101, 1119–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; King, R.B. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Han, Y.; Hou, X.; Ni, Y.; Wu, J. All are aromatic: A 3D globally aromatic cage containing five types of 2D aromatic macrocycles. Chem 2021, 7, 3442–3453. [Google Scholar] [CrossRef]
- Poater, J.; Viñas, C.; Bennour, I.; Escayola, S.; Solà, M.; Teixidor, F. Too Persistent to Give Up: Aromaticity in Boron Clusters Survives Radical Structural Changes. J. Am. Chem. Soc. 2020, 142, 9396–9407. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; De Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Silver and Copper Complexes with closo-Polyhedral Borane, Carborane and Metallacarborane Anions: Synthesis and X-ray Structure. Crystals 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Malinina, E.A.; Avdeeva, V.V.; Goeva, L.V.; Kuznetsov, N.T. Coordination compounds of electron-deficient boron cluster anions BnHn2− (n = 6, 10, 12). Russ. J. Inorg. Chem. 2010, 55, 2148–2202. [Google Scholar]
- Avdeeva, V.V.; Buzanov, G.A.; Malinina, E.A.; Kuznetsov, N.T.; Vologzhanina, A.V. Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion. Crystals 2020, 10, 389. [Google Scholar] [CrossRef]
- Avdeeva, V.; Malinina, E.; Kuznetsov, N. Boron cluster anions and their derivatives in complexation reactions. Coord. Chem. Rev. 2022, 469, 214636. [Google Scholar] [CrossRef]
- Korolenko, S.E.; Malinina, E.A.; Avdeeva, V.V.; Churakov, A.V.; Nefedov, S.E.; Kubasov, A.S.; Burlov, A.S.; Divaeva, L.N.; Zhizhin, K.Y.; Kuznetsov, N.T. Zinc(II) and cadmium(II) complexes with the decahydro-closo-decaborate anion and phenyl-containing benzimidazole derivatives with linker N N or C N group. Polyhedron 2020, 194, 114902. [Google Scholar] [CrossRef]
- Korolenko, S.E.; Kubasov, A.S.; Goeva, L.V.; Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Features of the formation of zinc(II) and cadmium(II) complexes with the inner-sphere and outer-sphere position of the decahydro-closo-decaborate anion in the presence of azaheterocyclic ligands. Inorg. Chim. Acta 2021, 520, 120315. [Google Scholar] [CrossRef]
- Korolenko, S.E.; Zhuravlev, K.P.; Tsaryuk, V.I.; Kubasov, A.S.; Avdeeva, V.V.; Malinina, E.A.; Burlov, A.S.; Divaeva, L.N.; Zhizhin, Y.K.; Kuznetsov, N.T. Crystal structures, luminescence, and DFT study of mixed-ligand Zn(II) and Cd(II) complexes with phenyl-containing benzimidazole derivatives with linker C = N or N = N group. J. Lumin. 2021, 237, 118156. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, Z.; Jiao, N.; Liu, L.; Zhang, S. B12H122−-Based Metal (Cu2+,Ni2+, Zn2+) complexes as hypergolic fuels with superior hypergolicity. Eur. J. Inorg. Chem. 2018, 2018, 981–986. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Kuznetsov, N.T. Multicenter interactions in lead(II) coordination compounds with BnHn2− (n = 6, 10, 12) cluster anions and their derivatives. Russ. J. Inorg. Chem. 2009, 54, 417–424. [Google Scholar] [CrossRef]
- Teixidor, F.; Vinas, C.; Demonceau, A.; Nuñez, R. Boron clusters: Do they receive the deserved interest? Pure Appl. Chem. 2003, 75, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, V.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Structural Diversity of Cationic Copper(II) Complexes with Neutral Nitrogen-Containing Organic Ligands in Compounds with Boron Cluster Anions and Their Derivatives (Review). Russ. J. Inorg. Chem. 2020, 65, 514–534. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Boron Cluster Anions [B10X10]2− (X = H, Cl) in Manganese(II) Complexation with 2,2′-Bipyridyl. Russ. J. Coord. Chem. 2019, 45, 295–300. [Google Scholar] [CrossRef]
- Belov, A.S.; Voloshin, Y.Z.; Pavlov, A.A.; Nelyubina, Y.V.; Belova, S.A.; Zubavichus, Y.V.; Avdeeva, V.V.; Efimov, N.N.; Malinina, E.A.; Zhizhin, K.Y.; et al. Solvent-induced encapsulation of cobalt(II) ion by a boron-capped tris-pyrazoloximate. Inorg. Chem. 2020, 59, 5845–5853. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Daletskii, V.A.; Lavrenova, L.G.; Trubina, S.V.; Erenburg, S.B.; Zhizhin, K.Y.; Kuzhetsov, N.T. Iron(II) closo-borate complexes with 1,2,4-triazole derivatives: Spin crossover in the iron(II) closo-borate complexes with tris(pyrazol-1-yl)methane. Russ. J. Inorg. Chem. 2013, 58, 650–656. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Lavrenova, L.G.; Korotaev, E.V.; Trubina, S.V.; Sheludyakova, L.A.; Petrov, S.A.; Zhizhin, K.Y.; Kuznetsov, N.T. High-Temperature Spin Crossover in Complexes of Iron(II) closo-Borates with 2,6-Bis(benzimidazol-2-yl)pyridine. Russ. J. Inorg. Chem. 2020, 65, 1687–1694. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Vologzhanina, A.V.; Sivaev, I.B.; Kuznetsov, N.T. Formation of oxidopolyborates in destruction of the [B11H14]− anion promoted by transition metals. Inorg. Chim. Acta 2020, 509, 119693. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Novikov, I.V.; Starodubets, P.A.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Formation of Polyborates during Dimerization of the closo-Decaborate Anion and Isomerization of the Octadecahydroeicosaborate Anion. Russ. J. Inorg. Chem. 2022, 67, 984. [Google Scholar] [CrossRef]
- Kozerozhets, I.; Panasyuk, G.; Semenov, E.; Voroshilov, I.; Avdeeva, V.; Buzanov, G.; Danchevskaya, M.; Kolmakova, A.; Malinina, E. A new approach to the synthesis of nanosized powder CaO and its application as precursor for the synthesis of calcium borates. Ceram. Int. 2021, 48, 7522–7532. [Google Scholar] [CrossRef]
- Sivaev, I.; Bregadze, V.I.; Kuznetsov, N. Derivatives of the closo-dodecaborate anion and their application in medicine. Bull. Acad. Sci. USSR Div. Chem. Sci. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Zhdanov, A.P.; Kuznetsov, N. Derivatives of closo-decaborate anion [B10H10]2− with exo-polyhedral substituents. Russ. J. Inorg. Chem. 2010, 55, 2089–2127. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo-decaborate anion chemistry. Collect. Czechoslov. Chem. Commun. 2010, 75, 1149–1199. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-Dodecaborate Anion [B12H12]2-: A Review. Collect. Czechoslov. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically Active Compounds Based on Membranotropic Cage Carriers–Derivatives of Adamantane and Polyhedral Boron Clusters (Review). Russ. J. Inorg. Chem. 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Dobrott, R.D.; Lipscomb, W.N. Reactions of B10H102− ion. Proc. Natl. Acad. Sci. USA 1962, 48, 729–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawthorne, M.F.; Shelly, K.; Li, F. The versatile chemistry of the [B20H18]2− ions: Novel reactions and structural motifs. Chem. Commun. 2002, 6, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Buzin, M.I.; Malinina, E.A.; Kuznetsov, N.T.; Vologzhanina, A.V. Reversible single-crystal-to-single-crystal photoisomerization of a silver(i) macropolyhedral borane. CrystEngComm 2015, 17, 8870–8875. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Buzin, M.I.; Dmitrienko, A.O.; Dorovatovskii, P.V.; Malinina, E.A.; Kuznetsov, N.T.; Voronova, E.D.; Zubavichus, Y.V.; Vologzhanina, A.V. Solid-State Reactions of Eicosaborate [B20 H18 ]2− Salts and Complexes. Chem.–A Eur. J. 2017, 23, 16819–16828. [Google Scholar] [CrossRef] [PubMed]
- Il’inchik, E.A.; Polyanskaya, T.M.; Drozdova, M.K.; Myakishev, K.G.; Ikorskii, V.N.; Volkov, V.V. Geometric, Spectroscopic, and Magnetic Characteristics of [Tris(1,10-phenanthroline)manganese](2+) 1,2;1′,2′-trans-Bis[nonahydro-closo-decaborate](2−) Bis(dimethylformamide). Russ. J. Gen. Chem. 2005, 75, 1545–1552. [Google Scholar] [CrossRef]
- Sirivardane, U.; Chu, S.S.C.; Hosmane, N.S.; Zhu, H.; Zhang, G. Bis[ethyl(ferrocenylmethyl)dimethylammonium] octadecahydroicosaborate(2-). Acta Cryst. 1989, C45, 333–336. [Google Scholar]
- Schaper, T.; Preetz, W. Hexahydro-closo-hexaborate as a ligand in coordination compounds: Syntheses and crystal structure of [M2(PPh3)2B6H6)] (M = Cu, Au). Chem. Ber. Recueil. 1997, 130, 405–408. [Google Scholar] [CrossRef]
- Malinina, E.A.; Drozdova, V.V.; Bykov, A.Y.; Belousova, O.N.; Polyakova, I.N.; Zhizhin, K.Y.; Kuznetsov, N.T. Complexes of gold clusters with the closo-borate anions B10H2−10 and B12H2−12. Dokl. Chem. 2007, 414, 137–139. [Google Scholar] [CrossRef]
- Exner, R.M.; Jenne, C.; Wegener, B. Electrochemical Synthesis of Triphenylphosphine Coinage Metal Complexes stabilized by closo-Dodecaborates [B12X12]2− (X = H, F, Cl, Br, I). Z. Anorg. Allg. Chem. 2021, 647, 500–506. [Google Scholar] [CrossRef]
- Jenne, C.; Wegener, B. Silver Salts of the Weakly Coordinating Anion [Me3 NB12 Cl11 ]-. Z. Anorg. Allg. Chem. 2018, 644, 1123–1132. [Google Scholar] [CrossRef]
- Wegener, M.; Huber, F.; Bolli, C.; Jenne, C.; Kirsch, S.F. Silver-Free Activation of Ligated Gold(I) Chlorides: The Use of [Me3NB12Cl11]− as a Weakly Coordinating Anion in Homogeneous Gold Catalysis. Chem.-Eur. J. 2015, 21, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Malinina, E.A.; Belousova, O.N.; Goeva, L.V.; Polyakova, I.N.; Kuznetsov, N.T. Behavior of dodecahydro-closo-dodecaborate anion B12H122− in reaction with Au(Ph3P)Cl. Russ. J. Inorg. Chem. 2011, 56, 524–529. [Google Scholar] [CrossRef]
- Guschlbauer, J.; Shaughnessy, K.H.; Pietrzak, A.; Chung, M.-C.; Sponsler, M.B.; Kaszyński, P. [closo-B10H8–1,10-(CN)2]2− as a conduit of electronic effects:comparative studies of Fe··· Fe communication in [{(η5–Cp)(dppe)Fe}2{µ2–(NC–X–CN)}]n+ (n = 0, 2). Organometallics 2021, 40, 2504. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Goeva, L.V.; Malinina, E.A.; Kuznetsov, N.T. Reactivity of boron cluster anions [B10H10]2−, [B10Cl10]2− and [B12H12]2− in cobalt(II)/cobalt(III) complexation with 1,10-phenanthroline. Inorg. Chim. Acta 2015, 428, 154–162. [Google Scholar] [CrossRef]
- Miller, H.C.; Miller, N.E.; Muetterties, E.L. Synthesis of Polyhedral Boranes. J. Am. Chem. Soc. 1963, 85, 3885–3886. [Google Scholar] [CrossRef]
- Chamberland, B.L.; Muetterties, E.L. Chemistry of Boranes. XVIII. Oxidation of B10H102- and Its Derivatives. Inorg. Chem. 1964, 3, 1450–1456. [Google Scholar] [CrossRef]
- Akhmadullina, N.S.; Churakov, A.V.; Retivov, V.M.; Sandu, R.A.; Shishilov, O.N. Gold(III) chloride and acetate complexes with bipyridine and phenanthroline. Russ. J. Coord. Chem. 2012, 38, 589–595. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Coordination chemistry of iron triad metals with organic N-donor ligands and boron cluster anions [B10H10]2−, [B12H12]2−, and [B10Cl10]2−: Complexation and accompanying processes. Russ. J. Inorg. Chem. 2017, 62, 1673–1702. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Polyakova, I.N.; Churakov, A.V.; Vologzhanina, A.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Complexation and exopolyhedral substitution of the terminal hydrogen atoms in the decahydro-closo-decaborate anion in the presence of cobalt(II). Polyhedron 2019, 162, 65–70. [Google Scholar] [CrossRef]
- Malinina, E.A.; Korolenko, S.E.; Zhdanov, A.P.; Avdeeva, V.V.; Privalov, V.I.; Kuznetsov, N.T. Metal-Promoted Exopolyhedral Substitution of Terminal Hydrogen Atoms in the Closo-Decaborate Anion [B10H10]2– in the Presence of Copper(II): Formation of the Substituted Derivative [2-B10H9OH]2−. J. Clust. Sci. 2020, 32, 755–763. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Polyakova, I.N.; Vologzhanina, A.V.; Goeva, L.V.; Buzanov, G.A.; Generalova, N.B.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. [Co(solv)6][B10H10] (solv = DMF and DMSO) for low-temperature synthesis of borides. Russ. J. Inorg. Chem. 2016, 61, 1125–1134. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Retivov, V.M.; Avdeeva, V.V.; Kuznetsov, N.T. Synthesis and Thermal Reduction of Complexes [NiLn][B10H10] (L = DMF, H2O, n = 6; L = N2H4, n = 3): Formation of Solid Solutions Ni3C1 − xBx. Russ. J. Inorg. Chem. 2020, 65, 126–132. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Efimov, N.N.; Kozerozhets, I.V.; Kuznetsov, N.T. Synthesis and Physicochemical Properties of Binary Cobalt(II) Borides. Thermal Reduction of Precursor Complexes [CoLn][B10H10] (L = H2O, n = 6; N2H4, n = 3). Russ. J. Inorg. Chem. 2019, 64, 1325–1334. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Efimov, N.N.; Kuznetsov, N.T. A New Method for Synthesis of Binary Borides with Desired Properties. Dokl. Chem. 2019, 487, 180–183. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeeva, V.V.; Vologzhanina, A.V.; Kubasov, A.S.; Akhmadullina, N.S.; Shishilov, O.N.; Malinina, E.A.; Kuznetsov, N.T. Gold(III) Complexation in the Presence of the Macropolyhedral Hydridoborate Cluster [B20H18]2−. Inorganics 2022, 10, 99. https://doi.org/10.3390/inorganics10070099
Avdeeva VV, Vologzhanina AV, Kubasov AS, Akhmadullina NS, Shishilov ON, Malinina EA, Kuznetsov NT. Gold(III) Complexation in the Presence of the Macropolyhedral Hydridoborate Cluster [B20H18]2−. Inorganics. 2022; 10(7):99. https://doi.org/10.3390/inorganics10070099
Chicago/Turabian StyleAvdeeva, Varvara V., Anna V. Vologzhanina, Alexey S. Kubasov, Nailya S. Akhmadullina, Oleg N. Shishilov, Elena A. Malinina, and Nikolay T. Kuznetsov. 2022. "Gold(III) Complexation in the Presence of the Macropolyhedral Hydridoborate Cluster [B20H18]2−" Inorganics 10, no. 7: 99. https://doi.org/10.3390/inorganics10070099
APA StyleAvdeeva, V. V., Vologzhanina, A. V., Kubasov, A. S., Akhmadullina, N. S., Shishilov, O. N., Malinina, E. A., & Kuznetsov, N. T. (2022). Gold(III) Complexation in the Presence of the Macropolyhedral Hydridoborate Cluster [B20H18]2−. Inorganics, 10(7), 99. https://doi.org/10.3390/inorganics10070099