Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand
Abstract
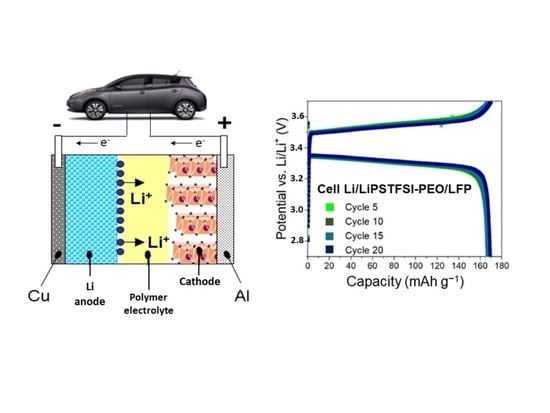
:1. Introduction
2. The Lithium Sulfonimide-Based Salts
3. Polymer Architecture
3.1. Copolymerization
3.2. Branched Architecture
4. Design of the Cathode
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Wen, K.; Chen, T.; Zhang, X.; Armand, M.; Chen, S. Recent progress in all-solid-state lithium batteries: The emerging strategies for advanced electrolytes and their interfaces. Energy Storage Mater. 2020, 31, 401–433. [Google Scholar] [CrossRef]
- Judez, X.; Eshetu, G.G.; Li, C.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Opportunities for rechargeable solid-state batteries based on Li-intercalation cathodes. Joule 2018, 2, 2208–2224. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Chabagno, J.M.; Duclot, M.J. Polymeric solid electrolytes. In Proceedings of the Extended Abstracts of the 2nd International Conference on Solid Electrolytes, St. Andrews, UK, 20–22 September 1978. [Google Scholar]
- Meng, N.; Lian, F.; Cui, G.L. Macromolecular design of lithium conductive polymer as electrolyte for solid-state lithium batteries. Small 2021, 17, 2005762. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From rocking chair—Li-ion to solid-state lithium batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Yang, J.F.; Dong, T.T.; Zhang, M.; Chai, J.C.; Dong, S.M.; Wu, T.Y.; Zhou, X.H.; Cui, G.L. Aliphatic polycarbonate-based solid-state polymer electrolytes for advanced lithium batteries: Advances and perspective. Small 2018, 14, 1800821. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Zeng, X.X.; Zhang, X.D.; Zuo, T.T.; Yan, M.; Yin, Y.X.; Shi, J.L.; Wu, X.W.; Guo, Y.G.; Wan, L.J. Engineering Janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.P.; Li, J.Y.; Wang, P.F.; Wang, W.P.; Shi, J.L.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef]
- Armand, M.; Gorecki, W.; Andreani, R. Second International Meeting on Polymer Electrolytes; Scrosati, B., Ed.; Elsevier: London, UK, 1990; p. 91. [Google Scholar]
- Syzdek, J.; Armand, M.; Marcinek, M.; Zalewska, A.; Zukowska, G.; Wieczorek, G. Detailed studies on the fillers modification and their influence on composite, poly(oxyethylene)-based polymeric electrolytes. Electrochim. Acta 2010, 55, 1314–1322. [Google Scholar] [CrossRef]
- Judez, X.; Piszcz, M.; Coya, E.; Li, C.; Aldalur, I.; Oteo, U.; Zhang, Y.; Zhang, W.; Rodriguez-Martinez, L.M.; Zhang, H.; et al. Stable cycling of lithium metal electrode in nanocomposite solid polymer electrolytes with lithium bis (fluorosulfonyl)imide. Solid State Ion. 2018, 318, 95–101. [Google Scholar] [CrossRef]
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. Challenges and issues facing lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Cui, Y. Cryogenic-electron microscopy is rapidly transforming battery research. Now, this powerful technique has been used to identify lithium hydride as a surprising culprit behind Li-ion battery failure. Nat. Energy 2018, 3, 1023–1024. [Google Scholar] [CrossRef]
- Qiao, L.; Oteo, U.; Zhang, Y.; Pena, S.R.; Martinez-Ibanez, M.; Santiago, A.; Cid, R.; Meabe, L.; Manzano, H.; Carrasco, J.; et al. Trifluoromethyl-free anion for highly stable lithium metal polymer batteries. Energy Storage Mater. 2020, 32, 225–233. [Google Scholar] [CrossRef]
- Qiao, L.; Oteo, U.; Matinez-Ibanez, M.; Santiago, A.; Cid, R.; Sanchez-Diez, E.; Lobato, E.; Meabe, L.; Armand, M.; Zhang, H. Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries. Nat. Mater. 2022, 21, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, U.; Oteo, X.; Judez, J.; Eshetu, G.G.; Martinez-Ibanez, M.; Carrasco, J.; Li, C.; Armand, M. Designer anion enabling solid-state lithium-sulfur batteries. Joule 2019, 3, 1689–1702. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Lakuntza, O.; Oteo, U.; Qiao, L.; Martinez Ibanez, M.; Zhu, H.; Carrasco, J.; Forsyth, M.; Armand, M. Suppressed Mobility of Negative Charges in Polymer Electrolytes with an Ether-Functionalized Anion. Angew. Chem. Int. Ed. 2019, 58, 12070–12075. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Xia, Y.; Feng, W.; Nie, J.; Hu, Y.-S.; Li, H.; Huang, X.; Chen, L.; Armand, M.; Zhou, Z. Impact of the functional group in the polyanion of single lithium-ion conducting polymer electrolytes on the stability of lithium metal electrodes. RSC Adv. 2016, 6, 32454–32461. [Google Scholar] [CrossRef]
- Devaux, D.; Liénafa, L.; Beaudoin, E.; Maria, S.; Phan, T.N.T.; Gigmes, D.; Giroud, E.; Davidson, P.; Bouchet, R. Comparison of single-ion-conductor block-copolymer electrolytes with polystyrene-TFSI and polymethacrylate-TFSI structural blocks. Electrochim. Acta 2018, 269, 250–261. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhou, C.; Zheng, L.; Cheng, P.; Nie, J.; Feng, W.; Hu, Y.S.; Li, H.; Huang, X.; et al. Single lithium-ion conducting polymer electrolytes based on a super-delocalized polyanion. Angew. Chem. Int. Ed. 2016, 55, 2521–2525. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Z.; Yuan, W.; Feng, W.; Nie, J.; Armand, M.; Huang, X.; Zhou, Z. Impact of negative charge delocalization on the properties of solid polymer electrolytes. ChemElectroChem 2021, 8, 1322–1328. [Google Scholar] [CrossRef]
- Zhang, H.; Oteo, U.; Zhu, H.; Judez, X.; Martinez- Ibanez, M.; Aldalur, I.; Sanchez-Diez, E.; Li, C.; Carrasco, J.; Forsyth, M.; et al. Enhanced lithium-ion conductivity of polymer electrolytes by selective introduction of hydrogen into the anion. Angew. Chem. Int. Ed. 2019, 58, 7829–7834. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Zheng, L.; Xu, F.; Feng, W.; Li, H.; Huang, X.; Armand, M.; Nie, J.; Zhou, Z. Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte. Electrochim. Acta 2014, 133, 529–538. [Google Scholar] [CrossRef]
- Tong, B.; Wang, P.; Ma, Q.; Wan, H.; Zhang, H.; Huang, X.; Armand, M.; Feng, W.; Nie, J.; Zhou, Z. Lithium fluorinated sulfonimide-based solid polymer electrolytes for Li||LiFePO4 cell: The impact of anionic structure. Solid State Ion. 2020, 358, 115519. [Google Scholar] [CrossRef]
- Yan, M.; Liang, J.Y.; Zuo, T.T.; Yin, Y.X.; Xin, S.; Tan, S.J.; Guo, Y.G.; Wan, L.J. Stabilizing polymer-lithium interface in a rechargeable solid battery. Adv. Funct. Mater. 2019, 30, 1908047. [Google Scholar] [CrossRef]
- Wang, W.; Fang, Z.; Zhao, M.; Peng, Y.; Zhang, J.; Guan, S. Solid polymer electrolytes based on the composite of PEO-LiFSI and organic ionic plastic crystal. Chem. Phys. Lett. 2020, 747, 137335. [Google Scholar] [CrossRef]
- Santiago, A.; Judez, X.; Castillo, J.; Garbayo, I.; Saenz de Buruaga, A.; Qiao, L.; Baraldi, G.; Coca-Clemente, J.A.; Armand, M.; Li, C.; et al. Improvement of lithium metal polymer batteries through a small dose of fluorinated salt. J. Phys. Chem. Lett. 2020, 11, 6133–6138. [Google Scholar] [CrossRef]
- Aldalur, I.; Martinez-Ibañez, M.; Piszcz, M.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Lowering the operational temperature of all-solid-state lithium polymer cell with highly conductive and interfacially robust solid polymer electrolytes. J. Power Sources 2018, 383, 144–149. [Google Scholar] [CrossRef]
- Aldalur, I.; Wang, X.; Santiago, A.; Goujon, N.; Echeverría, M.; Martínez-Ibáñez, M.; Piszcz, M.; Howlett, P.C.; Forsyth, M.; Armand, M.; et al. Nanofiber-reinforced polymer electrolytes toward room temperature solid-state lithium batteries. J. Power Sources 2020, 448, 227424. [Google Scholar] [CrossRef]
- Ye, W.; Zaheer, M.; Li, L.; Wang, J.; Xu, H.; Wang, C.; Deng, Y. Hyperbranched PCL/PS copolymer-based solid polymer electrolytes enable long cycle life of lithium metal batteries. J. Electrochem. Soc. 2020, 167, 110532. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Greene, G.W.; Zhou, Y.; Yoshizawa-Fujita, M.; Miyachi, Y.; Armand, M.; Forsyth, M.; Pringle, J.M.; Howlett, P.C. Organic ionic plastic crystal-based composite electrolyte with surface enhanced ion transport and its use in all-solid-state lithium batteries. Adv. Mater. Technol. 2017, 2, 1700046. [Google Scholar] [CrossRef]
- Nti, F.; Porcarelli, L.; Greene, G.W.; Zhu, H.; Makhlooghiazad, F.; Mecerreyes, D.; Howlett, P.C.; Forsyth, M.; Wang, X. The influence of interfacial interactions on the conductivity and phase behaviour of organic ionic plastic crystal/polymer nanoparticle composite electrolytes. J. Mater. Chem. A 2020, 8, 5350–5362. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Z.; Liao, Z.; Yang, L.; Hirano, S. Organic ionic plastic crystal-polymer solid electrolytes with high ionic conductivity and mechanical ability for solid-state lithium-ion batteries. Chem. Sel. 2018, 3, 12595–12599. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, F.; Li, J.; Liu, B.; Chen, X.; Dong, H.; Mao, Y.; Gu, W.; Xu, J.; Shen, Y.; et al. Composite solid electrolyte for solid-state lithium batteries workable at room temperature. ACS Appl. Energy Mater. 2020, 3, 12127–12133. [Google Scholar] [CrossRef]
- Zha, W.; Li, J.; Li, W.; Sun, C.; Wen, Z. Anchoring succinonitrile by solvent-Li+ associations for high-performance solid-state lithium battery. Chem. Eng. J. 2021, 406, 126754. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, X.; Wang, S.; Li, L.; Nan, C.W. Organic-organic composite electrolyte enables ultralong cycle life in solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 2020, 12, 24837–24844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xue, C.; Xin, C.; Lin, Y.; Shen, Y.; Li, L.; Nan, C.W. Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride)-based solid electrolytes. Adv. Mater. 2019, 31, 1806082. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, J.; Xie, W.; Chen, J.; Ke, X.; Ma, J.; Shi, Z. Effect of LiTFSI and LiFSI on cycling performance of lithium metal batteries using thermoplastic polyurethane/halloysite nanotubes solid electrolyte. Acta Metall. Sin. 2021, 34, 359–372. [Google Scholar] [CrossRef]
- Kuai, Y.; Wang, F.; Yang, J.; Lu, H.; Xu, Z.; Xu, X.; NuLi, Y.; Wang, J. Silica-nanoresin crosslinked composite polymer electrolyte for ambient-temperature all-solid-state lithium batteries. Mater. Chem. Front. 2021, 5, 6502–6511. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Girard, G.M.A.; Zhu, H.; MacFarlane, D.R.; Mecerreyes, D.; Armand, M.; Howlett, P.C.; Forsyth, M. Poly(ionic liquid)s-in-salt electrolytes with co-coordination-assisted lithium-ion transport for safe batteries. Joule 2019, 3, 2687–2702. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, W.; Zhou, Z.; Nie, J. Composite electrolytes of lithium salt/polymeric ionic liquid with bis(fluorosulfonyl)imide. Solid State Ion. 2014, 256, 61–67. [Google Scholar] [CrossRef]
- Kimura, K.; Yajima, M.; Tominaga, Y. A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem. Commun. 2016, 66, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; Tominaga, Y. Understanding electrochemical stability and lithium ion-dominant transport in concentrated poly(ethylene carbonate) electrolyte. ChemElectroChem 2018, 5, 4008–4014. [Google Scholar] [CrossRef]
- Tominaga, Y.; Yamazaki, K. Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem. Commun. 2014, 50, 4448–4450. [Google Scholar] [CrossRef] [PubMed]
- Judez, X.; Zhang, H.; Li, C.; Gonzalez-Marcos, J.A.; Zhou, Z.; Armand, M.; Rodriguez-Martinez, L.M. Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte for all solid-state Li-S cell. J. Phys. Chem. Lett. 2017, 8, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, G.G.; Judez, X.; Li, C.; Martinez-Ibanez, M.; Gracia, I.; Bondarchuk, O.; Carrasco, J.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Ultrahigh performance all solid-state lithium sulfur batteries: Salt anion’s chemistry-induced anomalous synergistic effect. J. Am. Chem. Soc. 2018, 140, 9921–9933. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; Zhang, Y.; Gonzalez-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Polymer-rich composite electrolytes for all-solid-state Li-S cells. J. Phys. Chem. Lett. 2017, 8, 3473–3477. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Judez, X.; Li, C.; Bondarchuk, O.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Lithium azide as an electrolyte additive for all-solid-state lithium–sulfur batteries. Angew. Chem. Int. Ed. 2017, 56, 15368–15372. [Google Scholar] [CrossRef] [PubMed]
- Aldalur, I.; Armand, M.; Zhang, H. Jeffamine-based polymers for rechargeable batteries. Batter. Supercaps 2019, 3, 30–46. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Wang, X.; Wu, H.; Feng, W.; Nie, J.; Yu, H.; Huang, X.; Armand, M.; Zhang, H.; Zhou, Z. Bis(fluorosulfonyl)imide-based electrolyte for rechargeable lithium batteries: A perspective. J. Power Sources Adv. 2022, 14, 100088. [Google Scholar] [CrossRef]
- Lopez-Aranguren, P.; Judez, X.; Chakir, M.; Armand, M.; Buannic, L. High voltage solid state batteries: Targeting high energy density with polymer composite electrolytes. J. Electrochem. Soc. 2020, 167, 020548. [Google Scholar] [CrossRef]
- Orue, A.; Arrese-Igor, M.; Cid, X.; Judez, X.; Gomez, N.; López del Amo, J.M.; Manalastas, W.; Srinivasan, M.; Rojviriya, C.; Armand, M.; et al. Enhancing the polymer electrolyte–Li metal interface on high-voltage solid-state batteries with Li-based additives inspired by the surface chemistry of Li7La3Zr2O12. J. Mater. Chem. A 2022, 10, 2352–2361. [Google Scholar] [CrossRef]
- Deng, P.L.; Zhang, H.; Feng, W.; Zhou, Z.; Armand, M.; Nie, J. Lithium (fluorosulfonyl) (pentafluoroethylsulfonyl)imide/poly (ethylene oxide) polymer electrolyte: Physical and electrochemical properties. Solid State Ion. 2019, 338, 161–167. [Google Scholar] [CrossRef]
- Martinez-Ibanez, M.; Sannchez-Diez, E.; Oteo, U.; Gracia, I.; Aldalur, I.; Eitouni, H.B.; Joost, M.; Armand, M.; Zhang, H. Anions with a dipole: Toward high transport numbers in solid polymer electrolytes. Chem. Mater. 2022, 34, 3451–3460. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Liénafa, L.; Bonnet, J.-P.; Phan, T.; Bertin, D.; Gibmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Meabe, L.; Lago, N.; Rubatat, L.; Li, C.; Müller, A.J.; Sardon, H.; Armand, M.; Mecerreyes, D. Polycondensation as a versatile synthetic route to aliphatic polycarbonates for solid polymer electrolytes. Electrochim. Acta 2017, 237, 259–266. [Google Scholar] [CrossRef]
- Meabe, L.; Huynh, T.V.; Mantione, D.; Porcarelli, L.; Li, C.; O’Dell, L.A.; Sardon, H.; Armand, M.; Forsyth, M.; Mecerreyes, D. UV-cross-linked poly(ethylene oxide carbonate) as free standing solid polymer electrolyte for lithium batteries. Electrochim. Acta 2019, 302, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Arrese-Igor, M.; Martinez-Ibanez, M.; del Amo, J.M.L.; Sanchez-Diez, E.; Shanmukaraj, D.; Dumond, E.; Armand, M.; Aguesse, F.; Lopez-Aranguren, P. Enabling double layer polymer electrolyte batteries: Overcoming the Li-salt interdiffusion. Energy Storage Mater. 2022, 45, 578–585. [Google Scholar] [CrossRef]
- Arrese-Igor, M.; Martinez-Ibanez, M.; Pavlenko, E.; Forsyth, M.; Zhu, H.; Armand, M.; Aguesse, F.; Lopez-Aranguren, P. Toward high-voltage solid-state Li-metal batteries with double-layer polymer electrolytes. ACS Energy Lett. 2022, 7, 1473–1480. [Google Scholar] [CrossRef]
- Martinez-Ibanez, M.; Sanchez-Diez, E.; Qiao, L.X.; Zhang, Y.; Judez, X.; Santiago, A.; Aldalur, I.; Carrasco, J.; Zhu, H.J.; Forsyth, M.; et al. Unprecedented improvement of single Li-ion conductive solid polymer electrolyte through salt additive. Adv. Funct. Mater. 2020, 30, 2000455. [Google Scholar] [CrossRef]
- Hao, S.-M.; Liang, S.; Sewell, C.D.; Li, Z.; Zhu, C.; Xu, J.; Lin, Z. Lithium-conducting branched polymers: New paradigm of solid-state electrolytes for batteries. Nano Lett. 2021, 21, 7435–7447. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhao, Y.; Dong, X.; Zhou, Y.; Wang, D.J. Frustrated crystallization and hierarchical self-assembly behaviour of comb-like polymers. Chem. Soc. Rev. 2013, 42, 2075–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Daigle, J.C.; Zaghib, K. Comprehensive review of polymer architecture for all-solid-state lithium rechargeable batteries. Materials 2020, 13, 2488. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Z.; Wu, H.; Nie, J.; Feng, W.; Yu, H.; Huang, X.; Armand, M.; Zhou, Z.; Zhang, H. Unprecedented impact of main chain on comb polymer electrolytes performances. ChemElectroChem 2022, 9, 202101590. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Rodehorst, U.; Streipert, B.; Wiemers-Meyer, S.; Jakelski, R.; Wagner, R.; Laskovic, I.C.; Winter, M. Learning from overpotentials in lithium-ion batteries: A case study on the LiNi1/3Co1/3Mn1/3O2 (NCM) cathode. J. Electrochem. Soc. 2016, 163, A2943–A2950. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem. Soc. Rev. 2019, 48, 465–4687. [Google Scholar] [CrossRef] [PubMed]
- Grillet, A.C.; Humplik, T.; Stirrup, E.K.; Roberts, S.A.; Barringer, D.A.; Snyder, C.M.; Janvrin, M.R.; Apblett, C.A. Conductivity degradation of plyvinylidene fluoride composite binder during cycling: Measurements and simulations for lithium-ion batteries. J. Electrochem. Soc. 2016, 163, A1859–A1871. [Google Scholar] [CrossRef] [Green Version]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer binders: Characterization and development toward aqueous electrode fabrication for sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Yang, Q.; Guo, X.; Yang, S.; Chen, A.; Liang, G.-J.; Zhi, C.-Y. Strategies of binder design for high-performance lithium-ion batteries: A mini review. Rare Met. 2022, 41, 745–761. [Google Scholar] [CrossRef]
- Li, S.; Lorandi, F.; Wang, H.; Liu, T.; Whitacre, J.F.; Matyjaszewski, K. Functional polymers for lithium metal batteries. Prog. Polym. Sci. 2021, 122, 101453. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J.-H.; Liu, Z.; Huang, X.; Krachkovskiy, S.A.; et al. Lithium polyacrylate (LiPAA) as an advanced binder and a passivating agent for high-voltage Li-ion batteries. Adv. Energy Mater. 2015, 5, 1501008. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Moon, J.; Ryou, M.-H.; Kim, S.-H.; Kim, J.-H.; Kim, J.-M.; Bang, J.; Lee, S.-Y. Amphiphilic bottlebrush polymeric binders for high-mass-loading cathodes in lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2102109. [Google Scholar] [CrossRef]
- Santiago, A.; Sanchez-Diez, E.; Oteo, U.; Aldalur, I.; Echeverria, M.; Armand, M.; Martinez-Ibanez, M.; Zhang, H. Single lithium ion conducting “binderlyte” for high-performing lithium metal batteries. Small 2022, 18, 2202027. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Völker, B.; Gan, Y.; McMeeking, R.M.; Kamlah, M. A micromechanical model for effective conductivity in granular electrode structures. Acta Mech. Sin. 2013, 29, 682–698. [Google Scholar] [CrossRef]






| SPE | σi (S cm−1) | Cathode | T (°C) | Capacity (mAh g−1) | C-Rate | Ref. |
|---|---|---|---|---|---|---|
| LiFSI/PEO (EO/Li+ = 20) | 3.3 × 10−4 | LFP | 80 | 146 (1st); 144 (20th) | 0.2 C (0.1) | [23] |
| LiFSI/PEO (EO/Li+ = 20) | 8.0 × 10−4 | LFP | 80 | 160 (1st); 144 (100th) | −0.3 | [24] |
| LiFNFSI/PEO (EO/Li+ = 20) | 5.0 × 10−4 | LFP | 80 | 120 (570th) | 0.1 C (0.1) | [11] |
| LiFSI/PEO + Mg3N2 (EO/Li+ = 20) | 1.7 × 10−4 | LFP | 60 | 150 (10th); 125 (50th) | 0.5 C (0.26) | [25] |
| LiFSI/PEO + N1222FSI | 2.1 × 10−4 | LFP | 50 | 159 (1st); 152 (90th) | 0.2 C (0.06) | [26] |
| LiTCM/PEO + LiFSI (1 wt.%) | 5.0 × 10−4 | LFP | 70 | 117 (20th); 108 (120th) | 0.3 C | [27] |
| LiFSI/Jeffamine (EO/Li+ = 20) | 2.0 × 10−4 | LFP | 50 | 120 (1st;) 50 (1st) | 0.1 C (0.1) | [28] |
| LiFSI/Jeffamine + PVDF (90 wt.%) (EO/Li + = 20) | 8.0 × 10−4 | LFP | 70 | 159 (1st); 130 (1st) | 0.1 C (0.1) | [29] |
| LiFSI/PEC (20 wt.%) | 1.6 × 10−5 | LFP | 30 | 120 (1st) | 0.05 C | [42] |
| LiFSI (30 wt.%)/PCL-PS (7:3 by wt) | 1.4 × 10−5 | LFP | 80 | 131 (1st); 99 (1200th) | 1 C | [30] |
| LiFSI/PVDF (60 wt.%) + PY12FSI (PY12+/Li + = 9:1) | 6.0 × 10−5 | LFP | 25 | ca 100 (1st) | 2 C (0.46) | [31] |
| LiFSI/PVDF (60 wt.%) + PY12FSI (PY12+/Li + = 9:1) | 1.4 × 10−5 | LFP | 50 | 130 (1st); 125 (100th) | 2 C (0.46) | [32] |
| LiFSI/P (VDF-HFP) (16 wt.%) + N1222FSI (64 wt.%) | 8.6 × 10−4 | LFP | 30 | 150 (1st); 140 (200th) | 0.2 C (0.21) | [33] |
| LiFSI/P (VDF-HFP) + PGCN (10 wt.%) | 1.4 × 10−4 | LFP | 26 | 129 (1st); 92 (400th) | 0.5 C | [34] |
| LiFSI/P (DADMA) FSI (DADMA+/Li+ = 2:3) | 0.7 × 10−4 | LFP | 80 | 160 (5th); 160 (30th) | −0.04 | [40] |
| LiFSI-SN/P (VDF-HFP) + ETPTA (15 wt.%) | 1.0 × 10−3 | LFP | 25 | 130 (10th); 129 (800th) | 1.0 C | [35] |
| LiFSI (40 wt.%)/PVDF + PAA (2 wt.%) | 1.3 × 10−4 | LCO | 30 | 120 (1st); 80 (1000th) | −0.088 | [36] |
| LiFSI/PVDF (60 wt.%) | 2.5 × 10−4 | LCO | 25 | 109 (1st) 119 (200th) | −0.15 | [37] |
| LiFSI/PEC (EC/Li+ = 5:6) | 2.7 × 10−5 | LMO | 60 | 95 (1st); 85 (10th) | −0.02 | [43] |
| LiFSI/P (DADMA) FSI (DADMA+/Li+ = 2:3) | 0.7 × 10−4 | NMC111 | 80 | 160 (5th); 130 (50th) | 0.05 C (0.06) | [40] |
| LiFSI/TPU-HNTs (22 wt.%) + PE | ~10−4 | NMC811 | 60 | 103 (3rd); 102 (300th) | 0.5 C | [38] |
| LiFSI/PVEC (16 wt.%) | 2.0 × 10−4 | NMC532 | 25 | 140 (1st); 55 (200th) | 0.5 C | [39] |
| LiFSI (16 wt.%)/PVEC + NR (VEC/NR = 4) | 1.6 × 10−4 | NMC532 | 25 | 145 (1st); 115 (200th) | 0.5 C | [39] |
| LiFSI/PEO (EO/Li+ = 20) | 9.0 × 10−4 | Sulfur | 70 | 420 (3rd); 550 (60th) | 0.1 C (0.16) | [46] |
| LiFSI/PEO + LiN3 (2 wt.%) (EO/Li+ = 20) | 5.0 × 10−4 | Sulfur | 70 | 850 (1st); 700 (30th) | 0.1 C (0.16) | [48] |
| LiFSI/PEO + LICGC (3 vol.%) (EO/Li+ = 20) | 8.0 × 10−4 | Sulfur | 70 | 784 (1st) | 0.1 C (0.16) | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.M. Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand. Inorganics 2022, 10, 110. https://doi.org/10.3390/inorganics10080110
Mauger A, Julien CM. Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand. Inorganics. 2022; 10(8):110. https://doi.org/10.3390/inorganics10080110
Chicago/Turabian StyleMauger, Alain, and Christian M. Julien. 2022. "Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand" Inorganics 10, no. 8: 110. https://doi.org/10.3390/inorganics10080110
APA StyleMauger, A., & Julien, C. M. (2022). Solid Polymer Electrolytes for Lithium Batteries: A Tribute to Michel Armand. Inorganics, 10(8), 110. https://doi.org/10.3390/inorganics10080110