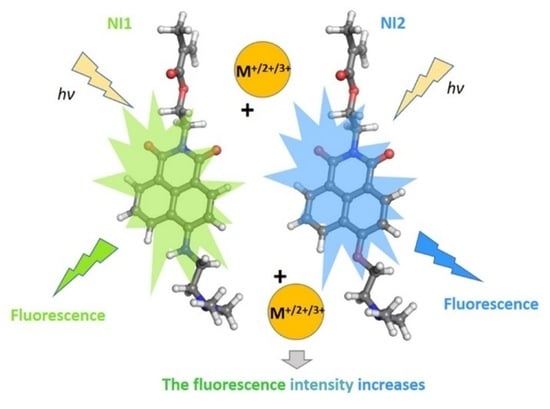
Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Compounds NI1 and NI2
2.2. Photophysical Characterization in Different Organic Solvents
2.3. Computational Studies
3. Materials and Methods
3.1. Synthesis of 1,8-Naphthalimides
3.2. Synthesis of 2-(6-(2-(N,N-Dimethylaminoethylamino)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate (NI1)
3.3. Synthesis of 2-(6-(2-(N,N-Dimethylaminoethoxy)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate (NI2)
3.4. Synthesis of 2-(6-bromo)-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl Methacrylate
3.5. Analysis
3.6. DFT Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Finney, N.S. Small-Molecule Fluorescent Probes and Their Design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-X.; Wang, X.-L.; Tusong; Xu, L.-H. Studies on the Synthesis and Spectral Properties of Novel 4-Benzofuranyl-1,8-Naphthalimide Derivatives. Dye. Pigment. 2005, 67, 27–33. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, M.; Shi, G.; Wang, L.; Yin, H.; Mei, C. A Novel Fluorescent Molecule Based on 1,8-Naphthalimide: Synthesis, Spectral Properties, and Application in Cell Imaging. Res. Chem. Intermed. 2010, 36, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Poteau, X.; Brown, A.I.; Brown, R.G.; Holmes, C.; Matthew, D. Fluorescence Switching in 4-Amino-1,8-Naphthalimides: “On–off–on” Operation Controlled by Solvent and Cations. Dye. Pigment. 2000, 47, 91–105. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Qi, J.; Liu, X.-Y.; He, H.-R.; Chen, J.-T.; Yang, G.-M. 4-Amino-1,8-Naphthalimide-Based Fluorescent Sensor with High Selectivity and Sensitivity for Zn2+ Imaging in Living Cells. Inorg. Chem. Commun. 2014, 43, 173–178. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New Fluorescent Chemosensors for Metal Ions in Solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Kelly, L.A.; Roll, M.; Joseph, J.; Seenisamy, J.; Barrett, J.; Kauser, K.; Warner, K.S. Solvent-Dependent Photophysics and Reactivity of Monomeric and Dimeric 4-Amino-1,8-Naphthalimides. J. Phys. Chem. A 2021, 125, 2294–2307. [Google Scholar] [CrossRef]
- Grabtchev, I.; Konstantinov, T.; Guittonneau, S.; Meallier, P. Photochemistry of Some 1,8-Naphthalic Anhydride Derivatives. Dye. Pigment. 1997, 35, 361–366. [Google Scholar] [CrossRef]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-Naphthalimide Derivatives as Dyes for Textile and Polymeric Materials: A Review. Fibers Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. Chemical Modification of Cotton Fabric with 1,8-Naphthalimide for Use as Heterogeneous Sensor and Antibacterial Textile. J. Photochem. Photobiol. A Chem. 2019, 382, 111924. [Google Scholar] [CrossRef]
- Staneva, D.; Angelova, S.; Grabchev, I. Spectral Characteristics and Sensor Ability of a New 1,8-Naphthalimide and Its Copolymer with Styrene. Sensors 2020, 20, 3501. [Google Scholar] [CrossRef]
- Jawale Patil, P.D.; Ingle, R.D.; Wagalgave, S.M.; Bhosale, R.S.; Bhosale, S.V.; Pawar, R.P.; Bhosale, S.V. A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection. Chemosensors 2019, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Mohr, G.J. Synthesis of Naphthalimide-Based Indicator Dyes with a 2-Hydroxyethylsulfonyl Function for Covalent Immobilisation to Cellulose. Sensors Actuators B Chem. 2018, 275, 439–445. [Google Scholar] [CrossRef]
- Callan, J.F.; de Silva, A.P.; Magri, D.C. Luminescent Sensors and Switches in the Early 21st Century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Grabchev, I.; Qian, X.; Xiao, Y.; Zhang, R. Novel Heterogeneous PET Fluorescent Sensors Selective for Transition Metal Ions or Protons: Polymers Regularly Labelled with Naphthalimide. New J. Chem. 2002, 26, 920–925. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and Colorimetric Chemosensors for Cations Based on 1,8-Naphthalimide Derivatives: Design Principles and Optical Signalling Mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Jain, N.; Kaur, N. A Comprehensive Compendium of Literature of 1,8-Naphthalimide Based Chemosensors from 2017 to 2021. Coord. Chem. Rev. 2022, 459, 214454. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Betcheva, R. Fluorescent Dendrimers as Sensors for Biologically Important Metal Cations. Curr. Med. Chem. 2012, 19, 4976–4983. [Google Scholar] [CrossRef]
- Temiz, H.T.; Boyaci, I.H.; Grabchev, I.; Tamer, U. Surface Enhanced Raman Spectroscopy as a New Spectral Technique for Quantitative Detection of Metal Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 339–347. [Google Scholar] [CrossRef]
- Oshchepkov, A.S.; Oshchepkov, M.S.; Oshchepkova, M.V.; Al-Hamry, A.; Kanoun, O.; Kataev, E.A. Naphthalimide-Based Fluorescent Polymers for Molecular Detection. Adv. Opt. Mater. 2021, 9, 2001913. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent Hydrogel–Textile Composite Material Synthesized by Photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Sali, S.; Guittonneau, S.; Grabchev, I. A Novel Blue Fluorescent Chemosensor for Metal Cations and Protons, Based on 1,8-Naphthalimide and Its Copolymer with Styrene. Polym. Adv. Technol. 2006, 17, 180–185. [Google Scholar] [CrossRef]
- Grabchev, I.; Dumas, S.; Chovelon, J.-M. Studying the Photophysical Properties of a Polymerizable 1,8-Naphthalimide Dye and Its Copolymer with Styrene as Potential Fluorescent Sensors for Metal Cations. Polym. Adv. Technol. 2008, 19, 316–321. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Grabchev, I.; Meallier, P.; Konstantinova, T.; Popova, M. Synthesis of Some Unsaturated 1,8-Naphthalimide Dyes. Dye. Pigment. 1995, 28, 41–46. [Google Scholar] [CrossRef]
- Grabchev, I.; Petkov, C.; Bojinov, V. 1,8-Naphthalimides as Blue Emitting Fluorophores for Polymer Materials. Macromol. Mater. Eng. 2002, 287, 904–908. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Lathan, W.A. Self-Consistent Molecular Orbital Methods. XIV. An Extended Gaussian-Type Basis for Molecular Orbital Studies of Organic Molecules. Inclusion of Second Row Elements. J. Chem. Phys. 1972, 56, 5255–5257. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 5 October 2022).










| Solvent | λA nm | ε L mol−1 cm−1 | λF nm | νA–νF cm−1 | ΦF |
|---|---|---|---|---|---|
| Acetonitrile | 436 | 12,400 | 517 | 3593 | 0.022 |
| N,N-dimethylformamide | 429 | 12,900 | 525 | 4262 | 0.010 |
| n-Butanol | 434 | 12,600 | 525 | 3994 | 0.018 |
| Ethanol | 438 | 12,700 | 536 | 4174 | 0.011 |
| Methanol | 439 | 12,400 | 538 | 4192 | 0.010 |
| Chloroform | 423 | 14,300 | 502 | 3720 | 0.716 |
| Dichloromethane | 424 | 14,600 | 504 | 3753 | 0.462 |
| Tetrahydrofuran | 420 | 14,400 | 500 | 3809 | 0.894 |
| λA nm | ε L mol−1 cm−1 | λF nm | νA–νF cm−1 | ΦF | |
|---|---|---|---|---|---|
| Acetonitrile | 366 | 12,300 | 422 | 3625 | 0.021 |
| N,N-dimethylformamide | 367 | 11,900 | 426 | 3773 | 0.015 |
| n-Butanol | 365 | 12,000 | 425 | 3867 | 0.019 |
| Ethanol | 368 | 12,200 | 438 | 4342 | 0.008 |
| Methanol | 369 | 12,400 | 439 | 4321 | 0.007 |
| Chloroform | 360 | 13,900 | 414 | 3623 | 0.624 |
| Dichloromethane | 360 | 13,600 | 413 | 3564 | 0.762 |
| Tetrahydrofuran | 358 | 13,400 | 410 | 3542 | 0.777 |
| Metal Ions | λA/nm | λF/nm | FE | Stokes Shift/cm−1 | ΦF |
|---|---|---|---|---|---|
| Ag+ | 428 | 515 | 8.90 | 3947 | 0.103 |
| Cu2+ | 429 | 516 | 14.29 | 3930 | 0.169 |
| Zn2+ | 429 | 520 | 4.20 | 4079 | 0.048 |
| Fe3+ | 428 | 510 | 34.25 | 3756 | 0.384 |
| Ni2+ | 429 | 521 | 8.40 | 4116 | 0.101 |
| Ca2+ | 428 | 524 | 1.5 | 4280 | 0.016 |
| Mg2+ | 428 | 524 | 1.9 | 4280 | 0.017 |
| Metal Ions | λA/nm | λF/nm | FE | Stokes Shift/cm−1 | ΦF |
|---|---|---|---|---|---|
| Ag+ | 365 | 416 | 9.40 | 3358 | 0.151 |
| Cu2+ | 365 | 415 | 16.23 | 3301 | 0.249 |
| Zn2+ | 367 | 422 | 6.32 | 3551 | 0.101 |
| Fe3+ | 365 | 412 | 38.05 | 3125 | 0.571 |
| Ni2+ | 366 | 415 | 9.9 | 3226 | 0.147 |
| Ca2+ | 365 | 425 | 1.8 | 3867 | 0.027 |
| Mg2+ | 365 | 426 | 1.9 | 3933 | 0.028 |
| Solvent | NI1 | NI2 | ||||
|---|---|---|---|---|---|---|
| calc. | exp. | μ | calc. | exp. | μ | |
| Toluene | 411 (0.34) | - | 9.16 | 364 (0.35) | - | 7.28 |
| Chloroform | 415 (0.34) | 423 | 9.99 | 366 (0.35) | 360 | 7.71 |
| Methanol | 419 (0.33) | 439 | 10.87 | 368 (0.34) | 369 | 8.18 |
| DMF | 420 (0.34) | 429 | 10.89 | 369 (0.35) | 367 | 8.19 |
| Water | 419 (0.33) | - | 10.97 | 368 (0.34) | - | 8.23 |
| Protonation Site | NI1 | NI2 | ||
|---|---|---|---|---|
| ΔG1 | ΔG78 | ΔG1 | ΔG78 | |
| N1 | 18.7 | 42.5 | 22.1 | 42.9 |
| N2/O | 10.9 | 20.9 | 34.8 | 43.9 |
| N3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Position | Mg2+ | Fe3+ |
|---|---|---|
| NI1 (N substituent) | −3.5 | −107.7 |
| NI1 (C-4 substituent) | −7.6 | −98.4 |
| NI2 (N substituent) | 7.0 | −102.7 |
| NI2 (C-4 substituent) | −8.7 | −93.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabchev, I.; Angelova, S.; Staneva, D. Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics 2023, 11, 47. https://doi.org/10.3390/inorganics11020047
Grabchev I, Angelova S, Staneva D. Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics. 2023; 11(2):47. https://doi.org/10.3390/inorganics11020047
Chicago/Turabian StyleGrabchev, Ivo, Silvia Angelova, and Desislava Staneva. 2023. "Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations" Inorganics 11, no. 2: 47. https://doi.org/10.3390/inorganics11020047
APA StyleGrabchev, I., Angelova, S., & Staneva, D. (2023). Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics, 11(2), 47. https://doi.org/10.3390/inorganics11020047