Organometallic Iridium Complexes with Glucose Based Phosphite Ligands
Abstract
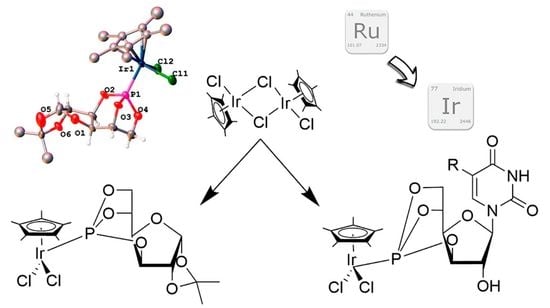
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Dichloro(1,2,3,4,5-pentamethylcyclopenta-1,3-diene)-[1-(3,5,6-bicyclophosphite-β-D-glucofuranosyl)uracil]iridium(III) (5)
4.2. Dichloro(1,2,3,4,5-pentamethylcyclopenta-1,3-diene)-[1-(3,5,6-bicyclophosphite-β-D-glucofuranosyl)thymine]iridium(III) (6)
4.3. Dichloro(1,2,3,4,5-pentamethylcyclopenta-1,3-diene)-[1-(3,5,6-bicyclophosphite-β-D-glucofuranosyl)-5-fluorouracil]iridium(III) (7)
4.4. Dichloro(1,2,3,4,5-pentamethylcyclopenta-1,3-diene)-[1-(3,5,6-bicyclophosphite-1,2-O-isopropylidene-α-d-glucofuranoside]iridium(III) (8)
4.5. Structure Determination
4.6. Cells and In Vitro Antiproliferative Assays
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abu-Surrah, A.; Kettunen, A.S.A.-S.A.M. Platinum Group Antitumor Chemistry: Design and development of New Anticancer Drugs Complementary to Cisplatin. Curr. Med. Chem. 2006, 13, 1337–1357. [Google Scholar] [CrossRef] [Green Version]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [Green Version]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Co-ord. Chem. Rev. 2017, 351, 2–31. [Google Scholar] [CrossRef]
- Ang, W.H.; Dyson, P.J. Classical and Non-Classical Ruthenium-Based Anticancer Drugs: Towards Targeted Chemotherapy. Eur. J. Inorg. Chem. 2006, 2006, 4003–4018. [Google Scholar] [CrossRef]
- Fish, R.H. A Bioorganometallic Chemistry Overview: From Cytochrome P450 Enzyme Metabolism of Organotin Compounds to Organorhodium-Hydroxytamoxifen Complexes with Potential Anti-Cancer Properties; A 37 Year Perspective at the Interface of Organometallic Chemistry and Biology. Aust. J. Chem. 2010, 63, 1505–1513. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Simpson, P.V.; Desai, N.M.; Casari, I.; Massi, M.; Falasca, M. Metal-Based Anticancer Compounds: Beyond Cisplatin. Future Med. Chem. 2019, 11, 119–135. [Google Scholar] [CrossRef]
- Tremlett, W.D.; Goodman, D.M.; Steel, T.R.; Kumar, S.; Wieczorek-Błauż, A.; Walsh, F.P.; Sullivan, M.P.; Hanif, M.; Hartinger, C.G. Design concepts of half-sandwich organoruthenium anticancer agents based on bidentate bioactive ligands. Co-ord. Chem. Rev. 2021, 445, 213950. [Google Scholar] [CrossRef]
- Shutkov, I.A.; Okulova, Y.N.; Tyurin, V.Y.; Sokolova, E.V.; Babkov, D.A.; Spasov, A.A.; Gracheva, Y.A.; Schmidt, C.; Kirsanov, K.I.; Shtil, A.A.; et al. Ru(III) Complexes with Lonidamine-Modified Ligands. Int. J. Mol. Sci. 2021, 22, 13468. [Google Scholar] [CrossRef]
- Parveen, S.; Hanif, M.; Leung, E.; Tong, K.K.H.; Yang, A.; Astin, J.; De Zoysa, G.H.; Steel, T.R.; Goodman, D.; Movassaghi, S.; et al. Anticancer organorhodium and -iridium complexes with low toxicity in vivo but high potency in vitro: DNA damage, reactive oxygen species formation, and haemolytic activity. Chem. Commun. 2019, 55, 12016–12019. [Google Scholar] [CrossRef]
- Qin, W.-W.; Pan, Z.-Y.; Cai, D.-H.; Li, Y.; He, L. Cyclometalated iridium(iii) complexes for mitochondria-targeted combined chemo-photodynamic therapy. Dalton Trans. 2020, 49, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P. Multinuclear biologically active Ru, Rh, Os and Ir arene complexes. Co-ord. Chem. Rev. 2021, 431, 213690. [Google Scholar] [CrossRef]
- Kacsir, I.; Sipos, A.; Bényei, A.; Janka, E.; Buglyó, P.; Somsák, L.; Bai, P.; Bokor, É. Reactive Oxygen Species Production Is Responsible for Antineoplastic Activity of Osmium, Ruthenium, Iridium and Rhodium Half-Sandwich Type Complexes with Bidentate Glycosyl Heterocyclic Ligands in Various Cancer Cell Models. Int. J. Mol. Sci. 2022, 23, 813. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Madji, R.; Voros, C.; Mazères, S.; Bijani, C.; Deraeve, C.; Cuvillier, O.; Gornitzka, H.; Maddelein, M.-L.; et al. N-Heterocyclic Carbene-Iridium Complexes as Photosensitizers for In Vitro Photodynamic Therapy to Trigger Non-Apoptotic Cell Death in Cancer Cells. Molecules 2023, 28, 691. [Google Scholar] [CrossRef]
- Hande, K. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Ludeman, M.S. The Chemistry of the Metabolites of Cyclophosphamide. Curr. Pharm. Des. 1999, 5, 627–643. [Google Scholar] [CrossRef]
- Maanen, M.; Smeets, C.; Beijnen, J. Chemistry, pharmacology and pharmacokinetics of N,N′,N′′ -triethylenethiophosphoramide (ThioTEPA). Cancer Treat. Rev. 2000, 26, 257–268. [Google Scholar] [CrossRef]
- Wardle, S.W.A.B.A.H.R.H.N.J.; Bligh, S.; Hudson, H. Organophosphorus Chemistry: Therapeutic Intervention in Mechanisms of Viral and Cellular Replication. Curr. Org. Chem. 2005, 9, 1803–1828. [Google Scholar] [CrossRef]
- Galanski, M.S.; Slaby, S.; Jakupec, M.A.; Keppler, B.K. Synthesis, Characterization, and in Vitro Antitumor Activity of Osteotropic Diam(m)ineplatinum(II) Complexes Bearing a N,N-Bis(phosphonomethyl)glycine Ligand. J. Med. Chem. 2003, 46, 4946–4951. [Google Scholar] [CrossRef]
- Dyson, P.J.; Sava, G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006, 16, 1929–1933. [Google Scholar] [CrossRef]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Hanessian, S. (Ed.) Preparative Carbohydrate Chemistry, 1st ed.; Marcel Dekker Inc.: New York, NY, USA, 1986; p. 650. [Google Scholar]
- Storr, T.; Thompson, K.H.; Orvig, C. Design of targeting ligands in medicinal inorganic chemistry. Chem. Soc. Rev. 2006, 35, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.; Renoux, M.; Lachaume, P.; Alziari, S. Bleomycin-induced double-strand breaks in mitochondrial DNA of Drosophila cells are repaired. Mutat. Res. Mol. Mech. Mutagen. 2008, 637, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, E.G.; Dragovich, T.; Hamm, J.; Langmuir, V.K.; Kroll, S.; Jung, D.T.; Colowick, A.B.; Tidmarsh, G.F.; Loehrer, P.J. A Phase 1 dose-escalation trial of glufosfamide in combination with gemcitabine in solid tumors including pancreatic adenocarcinoma. Cancer Chemother. Pharmacol. 2008, 61, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.A.; Randolph, J.K.; Yalowich, J.C.; Ritke, M.K.; Gewirtz, D.A. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol. Pharmacol. 1994, 45, 649–656. [Google Scholar]
- Nazarov, A.A.; Dyson, P.J. Metal Phosphorus Complexes as Antitumor Agents. In Phosphorus Compounds: Advanced Tools in Catalysis and Material Sciences, Peruzzini, M., Gonsalvi, L., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 445–461. [Google Scholar]
- Nazarov, A.A.; Baquié, M.; Nowak-Sliwinska, P.; Zava, O.; van Beijnum, J.R.; Groessl, M.; Chisholm, D.M.; Ahmadi, Z.; McIndoe, J.S.; Griffioen, A.W.; et al. Synthesis and characterization of a new class of anti-angiogenic agents based on ruthenium clusters. Sci. Rep. 2013, 3, srep01485. [Google Scholar] [CrossRef] [Green Version]
- Gonchar, M.R.; Matnurov, E.M.; Burdina, T.A.; Zava, O.; Ridel, T.; Milaeva, E.R.; Dyson, P.J.; Nazarov, A.A. Ruthenium(II)–arene and triruthenium-carbonyl cluster complexes with new water-soluble phopsphites based on glucose: Synthesis, characterization and antiproliferative activity. J. Organomet. Chem. 2020, 919, 121312. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug Profiling against SARS-CoV-2 Target Proteins Identifies Highly Potent Inhibitors of the S/ACE2 interaction and the Papain-like Protease PL pro. Chem. A Eur. J. 2021, 27, 17928–17940. [Google Scholar] [CrossRef]
- Lawson, B.; Slawin, A.M.Z.; Woollins, J.D. FUHQIL: (η5-pentamethylcyclopentadienyl)(triethyl phosphite)-dichloro-iridium and FUHQEH: (η5-pentamethylcyclopentadienyl)-(triphenyl phosphite)-dichloro-iridium toluene solvate. CSD Communication 2020. [CrossRef]
- Berger, I.; Hanif, M.; Nazarov, A.A.; Hartinger, C.G.; John, R.O.; Kuznetsov, M.L.; Groessl, M.; Schmitt, F.; Zava, O.; Biba, F.; et al. In Vitro Anticancer Activity and Biologically Relevant Metabolization of Organometallic Ruthenium Complexes with Carbohydrate-Based Ligands. Chem. A Eur. J. 2008, 14, 9046–9057. [Google Scholar] [CrossRef]
- Hanif, M.; Meier, S.M.; Kandioller, W.; Bytzek, A.; Hejl, M.; Hartinger, C.G.; Nazarov, A.A.; Arion, V.B.; Jakupec, M.A.; Dyson, P.J.; et al. From hydrolytically labile to hydrolytically stable RuII–arene anticancer complexes with carbohydrate-derived co-ligands. J. Inorg. Biochem. 2011, 105, 224–231. [Google Scholar] [CrossRef]
- Gonchar, M.R.; Ninin, F.S.; Milaeva, E.R.; Nazarov, A.A. Hydrolytically stable organometallic ruthenium complexes with glucose-based phosphite ligands. Russ. Chem. Bull. 2022, 71, 962–966. [Google Scholar] [CrossRef]
- Geisler, H.; Harringer, S.; Wenisch, D.; Urban, R.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Systematic Study on the Cytotoxic Potency of Commonly Used Dimeric Metal Precursors in Human Cancer Cell Lines. Chemistryopen 2022, 11, e202200019. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2003; p. 608. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Ir(1)-Cl(1) | 2.392(3) | O(3)-C(5) | 1.460(15) |
| P(1)-O(2) | 1.586(9) | O(4)-C(6) | 1.447(16) |
| P(1)-O(4) | 1.587(10) | O(5)-C(1) | 1.344(19) |
| P(1)-O(3) | 1.587(10) | O(6)-C(7) | 1.422(18) |
| O(1)-C(1) | 1.425(18) | O(6)-C(2) | 1.419(16) |
| O(1)-C(4) | 1.456(18) | ||
| P(1)-Ir(1)-Cl(1) | 89.68(12) | O(6)-C(2)-C(3) | 107.9(11) |
| O(2)-P(1)-O(4) | 103.4(6) | O(6)-C(2)-C(1) | 104.9(11) |
| O(2)-P(1)-O(3) | 103.2(5) | C(3)-C(2)-C(1) | 105.2(12) |
| O(4)-P(1)-O(3) | 96.2(5) | O(2)-C(3)-C(4) | 112.9(10) |
| O(2)-P(1)-Ir(1) | 113.4(3) | O(2)-C(3)-C(2) | 107.0(10) |
| O(4)-P(1)-Ir(1) | 122.1(4) | C(4)-C(3)-C(2) | 102.0(10) |
| O(3)-P(1)-Ir(1) | 115.7(4) | O(1)-C(4)-C(3) | 106.2(11) |
| C(1)-O(1)-C(4) | 108.6(12) | O(1)-C(4)-C(5) | 109.0(12) |
| C(3)-O(2)-P(1) | 117.7(8) | C(3)-C(4)-C(5) | 114.2(10) |
| C(5)-O(3)-P(1) | 105.4(8) | O(3)-C(5)-C(6) | 102.8(10) |
| C(6)-O(4)-P(1) | 110.9(10) | O(3)-C(5)-C(4) | 109.2(10) |
| C(1)-O(5)-C(7) | 111.1(15) | C(6)-C(5)-C(4) | 110.7(13) |
| C(7)-O(6)-C(2) | 110.4(12) | O(4)-C(6)-C(5) | 105.3(11) |
| O(5)-C(1)-O(1) | 116.8(15) | O(6)-C(7)-C(8) | 122(2) |
| O(5)-C(1)-C(2) | 103.9(15) | O(6)-C(7)-O(5) | 103.1(11) |
| O(1)-C(1)-C(2) | 107.9(13) | C(8)-C(7)-O(5) | 95(2) |
| Identification Code | 8 | Z(Z′) | 4(1) |
|---|---|---|---|
| Difractomer | Bruker D8 Quest with Photon III detector | Density (calculated) | 1.930 |
| Wavelength, Å | 0.71072 | Absorption coefficient | 63.46 |
| Empirical formula | C19H28Cl2IrO6P | F(000) | 1264 |
| Formula weight | 646.48 | θ range for data collection | 1.89 to 30.6 |
| Temperature, K | 122 | Reflections collected | 34,785 |
| Crystal system | Orthorhombic | Independent reflections | 6832 [R(int) = 0.0979] |
| Space group | P212121 | Observed reflections | 5483 |
| Unit cell dimensions | Completeness to θ max, % | 99.7 | |
| a (Å) | 9.8274(7) | Goodness-of-fit on F2 | 0.920 |
| b (Å) | 14.4958(10) | Final R indices [I > 2sigma(I)] | R1 = 0.0510, wR2 = 0.1325 |
| c (Å) | 15.6169(11) | R indices (all data) | R1 = 0.0711, wR2 = 0.1479 |
| Volume | 2224.7(3) | Largest diff. peak and hole | 2.200 and −1.515 e.Å −3 |
| Cell Lines | IC50, µM | ||||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | Cisplatin | |
| HCT116 | >100 | >100 | >100 | >100 | 13 ± 4 |
| A549 | >100 | >100 | >100 | >100 | 13 ± 3 |
| SW480 | >100 | >100 | >100 | >100 | 6.0 ± 0.3 |
| MCF7 | >100 | >100 | >100 | >100 | 30 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonchar, M.R.; Ninin, F.S.; Mazur, D.M.; Lyssenko, K.A.; Milaeva, E.R.; Nazarov, A.A. Organometallic Iridium Complexes with Glucose Based Phosphite Ligands. Inorganics 2023, 11, 124. https://doi.org/10.3390/inorganics11030124
Gonchar MR, Ninin FS, Mazur DM, Lyssenko KA, Milaeva ER, Nazarov AA. Organometallic Iridium Complexes with Glucose Based Phosphite Ligands. Inorganics. 2023; 11(3):124. https://doi.org/10.3390/inorganics11030124
Chicago/Turabian StyleGonchar, Maria R., Fedor S. Ninin, Dmitrii M. Mazur, Konstantin A. Lyssenko, Elena R. Milaeva, and Alexey A. Nazarov. 2023. "Organometallic Iridium Complexes with Glucose Based Phosphite Ligands" Inorganics 11, no. 3: 124. https://doi.org/10.3390/inorganics11030124
APA StyleGonchar, M. R., Ninin, F. S., Mazur, D. M., Lyssenko, K. A., Milaeva, E. R., & Nazarov, A. A. (2023). Organometallic Iridium Complexes with Glucose Based Phosphite Ligands. Inorganics, 11(3), 124. https://doi.org/10.3390/inorganics11030124