Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites
Abstract
:1. Introduction
2. Characterization
3. Beta Zeolite
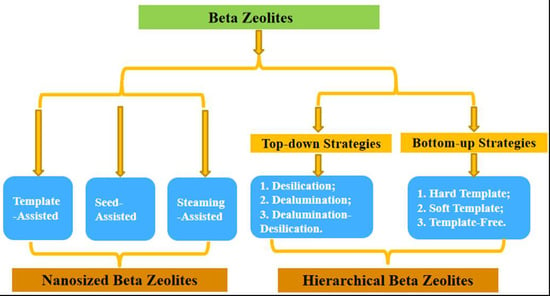
4. Nanosized Beta Zeolite
4.1. Template-Assisted Synthesis Method
4.2. Seed-Assisted Synthesis Method
4.3. Steam-Assisted Conversion Method
5. Hierarchical Beta Zeolites
5.1. Top-Down Method
5.1.1. Dealumination Method
5.1.2. Desilicication Method
5.1.3. Dealumination–Desilication Method
5.2. Bottom-Up Method
5.2.1. Hard Template Method
5.2.2. Soft Template Method
5.2.3. Template-Free Method
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDA | Structure-directing agent |
| OSDA | Organic structure-directing agent |
| PVB | Polyvinyl butyral |
| TEOS | Tetraethoxysilane |
| PDDA | Poly(diallyldimethylammonium chloride) |
| CTAB | Cetyltrimethylammonium bromide |
| N6-diphe | Gemini-type piperidine-based multiammonium surfactant |
| BJH | Barrett–Joyner–Halenda |
References
- Chen, L.H.; Sun, M.H.; Wang, Z.; Yang, W.M.; Xie, Z.K.; Su, B.L. Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.W.; Li, M.L.; Di, J.C.; Bai, P.; Yu, J.H. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.F.; Rei, G.Z.H.; Li, Z.Q.; Liu, X.L.; Fan, W.; Li, J.; Chen, X.B. Miguel A. Camblor, Fei-Jian Chen. A stable aluminosilicate zeolite with intersecting three-dimensional extra-large pores. Science 2021, 374, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef]
- Pan, X.L.; Jiao, F.; Miao, D.Y.; Bao, X.H. Oxide-zeolite-vased composite catalyst concept that enables syngas chemistry beyond Fischer–Tropsch synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef]
- Ramirez, A.; Gong, X.; Caglayan, M.; Nastase, S.A.F.; Abou-Hamad, E.; Gevers, L.; Cavallo, L.; Chowdhury, A.D.; Gascon, J. Selectivity descriptors for the direct hydrogenation of CO2 to hydrocarbons during zeolite-mediated bifunctional catalysis. Nat. Commun. 2021, 12, 5914. [Google Scholar] [CrossRef]
- Wang, X.R.; Zhou, T.; Zhang, P.; Yan, W.F.; Li, Y.G.; Peng, L.; Veerman, D.; Shi, M.Y.; Gu, X.H.; Kapteijn, F. High-silica CHA zeolite membrane with ultra-high selectivity and irradiation stability for krypton/xenon separation. Angew. Chem. Int. Ed. 2021, 60, 9032–9037. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Zhang, L.N.; Dai, W.L.; Chu, Y.Y.; Xu, J.; Wu, G.J.; Ye, M.; Deng, F.; Fan, W.B.; et al. Stabilizing the framework of SAPO-34 zeolite toward long-term methanol to olefins conversion. Nat. Commun. 2021, 12, 4661. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, X.F.; Hui, Y.; Wang, L.; Chen, W.; Qin, Y.C.; Wang, M.; Ma, J.B.; Chu, X.F.; Wang, Y.Q.; et al. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 2021, 372, 76–80. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J.H. Applications of zeolites in sustainable chemistry. Chemistry 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Centi, G.; Lanzafame, P.; Perathoner, S. Disruptive catalysis by zeolites. Catal. Sci. Technol. 2016, 6, 2485–2501. [Google Scholar] [CrossRef]
- Armor, J.N. A history of industrial catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Čejka, J.; Morris, R.E.; Serrano, D.P. Industrial applications of zeolite catalysis. Stud. Surf. Sci. Catal. 1994, 84, 2197–2219. [Google Scholar]
- Wang, J.J.; Chuang, Y.Y.; Hsu, H.Y.; Tsai, T.C. Toward industrial catalysis of zeolite for linear alkylbenzene synthesis: A mini review. Catal. Today 2017, 298, 109–116. [Google Scholar] [CrossRef]
- Yilmaz, B.; Müller, U. Catalytic applications of zeolites in chemical industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Zhao, D.P.; Wang, Y.N.; Chu, W.F.; Wang, X.Y.; Zhu, X.X.; Li, X.J.; Xie, S.J.; Wang, H.X.; Liu, S.L.; Xu, L.Y. Direct synthesis of hollow single-crystalline Beta zeolite using a small organic lactam as a recyclable hollow-directing agent. J. Mater. Chem. A 2019, 7, 10795–10804. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N. New method for the temperature-programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A review. Chem. Rec. 2013, 13, 432–455. [Google Scholar] [CrossRef]
- Blomsma, E.; Martens, J.A.; Jacobs, P.A. Mechanisms of heptane isomerization on bifunctional Pd/H-Beta zeolites. J. Catal. 1996, 159, 323–331. [Google Scholar] [CrossRef]
- Corma, A.; Gomez, V.; Martinez, A. Zeolite Beta as a catalyst for alkylation of isobutane with 2-butene. Influence of synthesis conditions and process variables. Appl. Catal. A Gen. 1994, 119, 83–96. [Google Scholar] [CrossRef]
- Derouane, E.G.; Schemidt, I.; Lachas, H.; Christensen, C.J.H. Improved performance of nano-size H-BEA zeolite catalysts for the Friedel-Crafts Acetylation of anisole by acetic anhydride. Catal. Lett. 2004, 95, 13. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E.; Ferna’ndez, J.A. Catalytic conversion of polyolefins into fuels over zeolite Beta. Polym. Degrad. Stab. 2000, 69, 11–16. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, X.; Zhang, Y.; Tang, Y. Future of nano-/hierarchical zeolites in catalysis: Gaseous phase or liquid phase system. Catal. Sci. Technol. 2015, 5, 772. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Qu, H.; Ma, Y.; Li, B.; Wang, L. Hierarchical zeolites: Synthesis, structural control, and catalytic applications. Emergent Mater. 2020, 3, 225. [Google Scholar] [CrossRef]
- Pan, H.; Peng, R.S.; Zhu, Z.G.; Xu, H.; He, M.Y.; Wu, P. Highly Hydrophilic Ti-Beta Zeolite with 24. Ti-Rich Exterior as Efficient Catalyst for Cyclohexene Epoxidation. Catalysts 2022, 12, 434. [Google Scholar] [CrossRef]
- Shen, Z.; Kong, L.; Zhang, W.; Gu, M.Y.; Xia, M.; Zhou, X.F.; Zhang, Y.L. Surface amino-functionalization of Sn-Beta zeolite catalyst for lactic acid production from glucose. RSC Adv. 2019, 9, 18989. [Google Scholar] [CrossRef]
- Cheng, D.J.; Meng, X.J. Recent Advances of Beta Zeolite in the Volatile Organic Compounds (VOCs) Elimination by the Catalytic Oxidations. Chem. Res. Chin. Univ. 2022, 38, 716–722. [Google Scholar] [CrossRef]
- Huang, G.; Ji, P.; Xu, H.; Jiang, J.G.; Chen, L.; Wu, P. Fast synthesis of hierarchical Beta zeolites with uniform nanocrystals from layered silicate precursor. Microporous Mesoporous Mater. 2017, 248, 30–39. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.D.; Sukenaga, S.; Ando, M.; Shibata, H.; Okubo, T.; Wakihara, T. Ultrafast synthesis of *BEA zeolite without the aid of aging pretreatment. Microporous Mesoporous Mater. 2018, 268, 1–8. [Google Scholar] [CrossRef]
- Larlus, O.; Mintova, S.; Wilson, S.T.; Willis, R.R.; Abrevaya, H.; Bein, T. A powerful structure-directing agent for the synthesis of nanosized Al-and high-silica Beta zeolite in alkaline medium. Microporous Mesoporous Mater. 2011, 142, 17–25. [Google Scholar] [CrossRef]
- Zhao, D.P.; Chu, W.F.; Wang, Y.N.; Zhu, X.X.; Li, X.J.; Xie, S.J.; An, J.; Xin, W.J.; Liu, S.L.; Xu, L.Y. Organic promoter-driven fast synthesis of Beta zeolite and its acceleration mechanism. J. Mater. Chem. A 2018, 6, 24614–24624. [Google Scholar] [CrossRef]
- Xiao, F.S.; Wang, L.F.; Yin, C.Y.; Lin, K.F.; Di, Y.; Li, J.X.; Xu, R.R.; Su, D.S.; Schlögl, R.; Yokoi, T.; et al. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem. 2006, 45, 3090–3093. [Google Scholar] [CrossRef]
- Song, J.W.; Ren, L.M.; Yin, X.Y.; Ji, Y.Y.; Wu, Z.F.; Li, J.X.; Xiao, F.S. Stable, Porous, and bulky particles with high external surface and large pore volume from self-assembly of zeolite nanocrystals with cationic polymer. J. Phys. Chem. C 2008, 112, 8609–8613. [Google Scholar] [CrossRef]
- Wang, B.Y.; Yan, X.M.; Zhang, H.Y. Interaction between phosphorus and zeolite/binder: A realumination study on beta zeolites. Microporous Mesoporous Mater. 2021, 312, 110735. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, B.; Huang, J.; Zhang, K.; Li, F.E.; Xi, H.X. Cationic surfactant-directed synthesis of hollow Beta zeolite with hierarchical structure. Inorg. Chem. Commun. 2019, 107, 107468. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, crystallization mechanism, and applications. Chem. Mater. 2005, 17, 2494–2513. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Liu, C.; Zhang, Y.H.; Ren, N.; Tang, Y. Microwave-assisted hydrothermal synthesis of nanozeolites with controllable size. Microporous Mesoporous Mater. 2009, 119, 306–314. [Google Scholar] [CrossRef]
- Vuong, G.T.; Do, T.O. A new route for the synthesis of uniform nanozeolites with hydrophobic external surface in organic solvent medium. J. Am. Chem. Soc. 2007, 129, 3810–3811. [Google Scholar] [CrossRef]
- Mintova, S.; Gilson, J.P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693–6703. [Google Scholar] [CrossRef]
- Shao, J.L.; Rim, C.J.; van de Poll, R.; Kosinov, N.; Hensen, E.J.M. Facile synthesis of nanosized mordenite and Beta zeolites with improved catalytic performance: Non-surfactant diquaternary ammonium compounds as structure-directing agents. Inorg. Chem. Front. 2022, 9, 3200–3216. [Google Scholar]
- Martínez, F.R.; Paris, C.; Martínez Armero, M.E.; Martínez, C. High-silica nanocrystalline Beta zeolites: Efficient synthesis and catalytic application. Chem. Sci. 2016, 7, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Wu, Q.M.; Lei, C.; Han, S.C.; Zhu, Q.Y.; Maurer, S.; Dai, D.; Parvulescu, A.N.; Müller, U.; Meng, X.J.; et al. An efficient, rapid, and non-centrifugation synthesis of nanosized zeolites by acceleration the nucleation rate. J. Mater. Chem. A 2018, 6, 21156–21161. [Google Scholar] [CrossRef]
- Kecht, J.; Mintova, S.; Bein, T. Nanosized Zeolites Templated by Metal-Amine Complexes. Chem. Mater. 2007, 19, 1203–1205. [Google Scholar] [CrossRef]
- Kecht, J.; Mintova, S.; Bein, T. Exceptionally small colloidal zeolites templated by Pd and Pt amines. Langmuir 2008, 24, 4310–4315. [Google Scholar] [CrossRef]
- Dai, H.; Shen, Y.; Yang, T. Finned zeolite catalysts. Nat. Mater. 2020, 19, 1074–1080. [Google Scholar] [CrossRef]
- Li, C.; Moliner, M.; Corma, A. Building zeolites from precrystallized units: Nanoscale architecture. Angew. Chem. Int. Ed. 2018, 57, 15330–15353. [Google Scholar] [CrossRef]
- Martin, N.; Li, Z.; Martinez-Triguero, J. Nanocrystalline SSZ-39 zeolite as an efficient catalyst for the methanol-to-olefin (MTO) process. Chem. Commun. 2016, 52, 6072–6075. [Google Scholar] [CrossRef]
- Xu, H.; Chen, W.; Wu, Q. Transformation synthesis of aluminosilicate SSZ-39 zeolite from ZSM-5 and Beta zeolite. J. Mater. Chem. A 2019, 7, 4420–4425. [Google Scholar] [CrossRef]
- Majano, G.; Darwiche, A.; Mintova, S.; Valtchev, V. Seed-induced crystallization of nanosized Na-ZSM-5 crystals. Ind. Eng. Chem. Res. 2009, 48, 7084–7091. [Google Scholar] [CrossRef]
- Zhang, H.D.; Wu, C.Y.; Song, M.X.; Lu, T.T.; Wang, W.D.; Wang, Z.W.; Yan, W.F.; Cheng, P.; Zhao, Z. Accelerated synthesis of Al-rich zeolite Beta via different radicalized seeds in the absence of organic templates. Microporous Mesoporous Mater. 2021, 310, 110633. [Google Scholar] [CrossRef]
- Zhen, L.; Hu, L.; Yi, W.; Yu, W.; Peng, P.; Xin, M.L. Seeds induced Beta zeolite synthesis with low SDA for n-heptane catalytic cracking reaction. Catal. Today 2022, 405–406, 235–241. [Google Scholar]
- Zhu, P.; Li, P.; Riisager, A. Sn-Beta catalyzed transformations of sugars-advances in catalyst and applications. Catalysts 2022, 12, 405. [Google Scholar] [CrossRef]
- Miao, S.S.; She, P.; Chang, X.Y.; Zhao, C.; Sun, Y.T.; Lei, Z.Y.; Sun, S.S.; Zhang, W.X.; Jia, M.J. Synthesis of Beta nanozeolite aggregates with hierarchical pores via steam-assisted conversion of dry gel and their catalytic properties for Friedel-Crafts acylation. Microporous Mesoporous Mater. 2022, 334, 111777. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Li, L.; Chen, M.Y.; Li, J.Y.; Yu, J.H. Steam-assisted crystallization of highly dispersed nanosized hierarchical zeolites from solid raw materials and their catalytic performance in lactide production. Chem. Sci. 2022, 13, 8052–8059. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, S.; Zhang, Q. Breaking the Si/Al limit of nanosized β zeolites: Promoting catalytic production of lactide. Chem. Mater. 2019, 32, 751–758. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Serrano, D.P.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Bolshakov, A.; Kosinov, N.; Romero Hidalgo, D.E.; Mezari, B.; van Hoof, A.J.F.; Hensen, E.J.M. Mild dealumination of template-stabilized zeolites by NH4F. Catal. Sci. Technol. 2019, 9, 4239–4247. [Google Scholar] [CrossRef]
- Feng, R.F.; Yan, X.L.; Hu, X.Y.; Wang, Y.L.; Zheng, L.Z.; Hou, K.; Lin, J.W. Hierarchical ZSM-5 zeolite designed by combining desilication and dealumination with related study of n-heptane cracking performance. J. Porous Mater. 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Yang, S.T.; Yu, C.X.; Yu, L.L.; Miao, S.; Zou, M.M.; Jin, C.Z.; Zhang, D.Z.; Xu, L.Y.; Huang, S.J. Bridging Dealumination and Desilication for the Synthesis of Hierarchical MFI Zeolites. Angew. Chem. Int. Ed. 2017, 56, 12553–12556. [Google Scholar] [CrossRef] [PubMed]
- Donk, S.V.; Janssen, A.H.; Bitter, J.H.; Jong, K.P.D. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Wang, W.N.; Zhang, W.; Wen, X.D.; Shen, B.J. Mild-acid-assisted thermal or hydrothermal dealumination of Beta zeolite, its regulation to Al distribution and catalytic cracking performance to hydrocarbons. J. Catal. 2018, 362, 94–105. [Google Scholar] [CrossRef]
- Suarez, N.; Perez-Pariente, J.; Márquez-Álvarez, C.; Casas, M.C.; Mayoral, A.; Moreno, A. Preparation of mesoporous Beta zeolites by fluoride treatment in liquid phase. Textural, acid and catalytic properties. Microporous Mesoporous Mater. 2019, 284, 296–303. [Google Scholar] [CrossRef]
- Wan, W.; Fu, T.; Qi, R.; Shao, J.; Li, Z. Coeffect of Na+ and tetrapropylammonium (TPA+) in alkali treatment on the fabrication of mesoporous ZSM-5 catalyst for methanol-to-hydrocarbons reactions. Ind. Eng. Chem. Res. 2016, 55, 13040. [Google Scholar] [CrossRef]
- Li, X.Y.; Gaitan, A.H.G.; Kokuryo, S.; Sumi, T.; Kitamura, H.; Koji Miyake, K.; Uchida, Y.; Nishiyama, N. Hierarchical zeolites with high hydrothermal stability prepared via desilication of OSDA-occluded zeolites. Microporous Mesoporous Mater. 2022, 344, 112096. [Google Scholar] [CrossRef]
- Zhang, K.; Fernandez, S.; Converse, E.S.; Kobaslija, S. Exploring the impact of synthetic strategies on catalytic cracking in hierarchical Beta zeolites via hydrothermal desilication and organosilane-templated synthesis. Catal. Sci. Technol. 2020, 10, 4602–4611. [Google Scholar] [CrossRef]
- Zhu, P.; Meier, S.; Saravanamurugan, S.; Riisager, A. Modification of commercial Y zeolites by alkaline-treatment for improved performance in the isomerization of glucose to fructose. Mol. Catal. 2021, 510, 111686. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Perez-Ramirez, J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Groenet, J.C.; Villaescusa, L.A. Mesoporous Beta zeolite obtained by desilication. Microporous Mesoporous Mater. 2008, 114, 93–102. [Google Scholar] [CrossRef]
- Tian, F.P.; Cao, C.X.; Hu, M. Urea solution treatment: A facile and moderate approach to achieve hierarchical Beta zeolite. Catal. Commun. 2016, 83, 66–69. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Mesopore Formation in USY and Beta Zeolites by Base Leaching: Selection Criteria and Optimization of Pore-Directing Agents. Cryst. Growth Des. 2012, 12, 3123–3132. [Google Scholar] [CrossRef]
- Zhang, K.; Fernandez, S.; Lawrence, J.A., III; Ostraat, M.L. Organotemplate-free Beta zeolites: From zeolite synthesis to hierarchical structure Creation. ACS Omega 2018, 3, 18935–18942. [Google Scholar] [CrossRef]
- Leng, K.Y.; Li, X.L.; Ye, G. Ti-containing hierarchical Beta with highly active sites for deep desulfurization of fuels under mild conditions. Catal. Sci. Technol. 2016, 6, 7615–7622. [Google Scholar] [CrossRef]
- Xu, S.L.; Zhang, M.Y.; Guo, S.J.; Li, M.R.; Huang, Q.M.; Chen, X.H. Synthesis of Hierarchically Porous Zeolite Ti-MWW with Different Hard Templates and Their Application in Allyl Alcohol Conversion. Catal. Lett. 2020, 150, 209–221. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.; Zhou, F.; Ma, H.X.; Qiao, K.; Lu, X.Y.; Fu, J.; Huang, H. Catalytic fast pyrolysis of cellulose to aromatics over hierarchical nanocrystalline ZSM-5 zeolites prepared using sucrose as a template. Catal. Commun. 2018, 110, 102–105. [Google Scholar] [CrossRef]
- Li, D.B.; Chen, Y.M.; Hu, J.P.; Deng, B.Q.; Cheng, X.W.; Zhang, Y. Synthesis of Hierarchical Chabazite Zeolite via Interzeolite Transformation of Coke-containing Spent MFI. Appl. Catal. B Environ. 2020, 270, 118881. [Google Scholar] [CrossRef]
- Karimi, H.; Towfighi, J.; Akhgar, S. Synthesis of hierarchical SAPO-34 zeolites with tuned mesopore structure for methanol to olefins (MTO) reaction using polyethylene glycol as a soft template. J. Sol-Gel Sci. Technol. 2021, 100, 286–298. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Zhang, Y.C.; Zhu, K.K.; Qian, G.; Zhou, X.G. Carbon nanotubes as transient inhibitors in steam-assisted crystallization of hierarchical ZSM-5 zeolites. Mater. Lett. 2015, 159, 466–469. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Meng, L.; Jiang, N. Synthesis of uniform mesoporous ZSM-5 using hydrophilic carbon as a hard template. Mater. Chem. Phys. 2016, 177, 112–117. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Qi, S.Q.; Xu, L.L.; Shi, G.S.; Ding, Y.H.; Yan, X.; Huang, Y.; Geng, J.X. Graphene oxide facilitates solvent-free synthesis of well-dispersed, Faceted zeolite crystals. Angew. Chem. Int. Ed. 2017, 56, 14090–14095. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Lavorato, C.; Mastropietro, T.F.; Argurio, P.; Drioli, E.; Poerio, T. Preparation of Pd-loaded hierarchical FAU nembranes and testing in acetophenone hydrogenation. Molecules 2016, 21, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.J.H.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous Zeolite Single Crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Zhang, L.C.; Sun, X.B.; Pan, M.; Yang, X.N.; Liu, Y.C.; Sun, J.H.; Ma, J.H.; Li, W.L.; Li, R.F. Interfacial effects between carbon nanotube templates and precursors on fabricating a wall-crystallized hierarchical pore system in zeolite crystals. J. Mater. Sci. 2020, 55, 10412–10426. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Wang, Y.; Ma, J.H.; Li, R.F. Preparation of Hierarchical ZSM-5 Zeolites by in-situ Crystallization of Mesoporous Carbon-Silica Composite. ChemistrySelect 2020, 5, 14130–14135. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.F.; Shang, W.J.; Su, X.P.; Hao, Q.Q.; Chen, H.Y.; Ma, X.X. Synthesis, characterization, and catalytic application of hierarchical SAPO-34 zeolite with three-dimensionally ordered mesoporous-imprinted structure. Microporous Mesoporous Mater. 2017, 252, 10–16. [Google Scholar] [CrossRef]
- Tao, Y.S.; Kanoh, H.; Kaneko, K. ZSM-5 Monolith of Uniform Mesoporous Channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovation in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Sun, M.H.; Chen, L.H.; Yu, S.; Li, Y.; Zhou, X.G.; Hu, Z.Y.; Sun, Y.H.; Su, B.L. Micron-sized zeolite Beta single crystals featuring intracrystal interconnected ordered macro-meso-microporosity displaying superior catalytic performance. Angew. Chem. Int. Ed. 2020, 59, 19582–19591. [Google Scholar] [CrossRef]
- Hui, Y.C.; James, W.; Xue, Y.Z.; Pyung-Soo, L.; Zhuo, P.W.; Wei, F.; Michael, T. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar]
- Schmidt, I.; Madsen, C.; Jacobsen, C.J.H. Confined Space Synthesis. A Novel Route to Nanosized Zeolites. Inorg. Chem. 2003, 39, 2279–2283. [Google Scholar] [CrossRef]
- Jin, J.J.; Ye, X.X.; Shi, J.L. Synthesis of mesoporous Beta and Sn-Beta zeolites and their catalytic performances. Dalton Trans. 2014, 43, 8196. [Google Scholar] [CrossRef]
- Zhu, H.B.; Liu, Z.C.; Kong, D.J.; Xie, Z.K. Synthesis and Catalytic performances of mesoporous zeolites templated by polyvinyl butyral gel as the mesopore directing agent. J. Phys. Chem. C 2008, 112, 17257–17264. [Google Scholar] [CrossRef]
- Zhang, W.M.; Ming, W.X.; Hu, S.F.; Qin, B.; Ma, J.H.; Li, R.F. A feasible one-step synthesis of hierarchical zeolite Beta with uniform nanocrystals via CTAB. Materials 2018, 11, 651–662. [Google Scholar] [CrossRef]
- Du, S.T.; Li, F.; Sun, Q.M.; Wang, N.; Jia, M.J.; Yu, J.H. A green surfactant-assisted synthesis of hierarchical TS-1 zeolites with excellent catalytic properties for oxidative desulfurization. Chem. Commun. 2016, 52, 3368–3371. [Google Scholar] [CrossRef]
- Xu, H.; Lei, C.; Wu, Q.M.; Zhu, Q.Y.; Meng, X.J.; Dai, D.; Maurer, S.; Parvulescu, A.N.; Müller, U.; Xiao, F.S. Organosilane surfactantassisted synthesis of mesoporous SSZ-39 zeolite with enhanced catalytic performance in the methanol-to-olefins reaction. Front. Chem. Sci. Eng. 2020, 14, 267–274. [Google Scholar] [CrossRef]
- Xu, S.M.; Zhang, X.X.; Cheng, D.G.; Chen, F.Q.; Ren, X.H. Effect of hierarchical ZSM-5 zeolite crystal size on diffusion and catalytic performance of n-heptane cracking. Front. Chem. Sci. Eng. 2018, 12, 780–789. [Google Scholar] [CrossRef]
- Guo, D.X.; Shi, C.X.; Zhao, H.; Chen, R.; Chen, S.H.; Sun, P.C.; Chen, T.H. Polyacrylic acid as mesoscale template for synthesis of MFI zeolite with plentiful intracrystalline mesopores. Microporous Mesoporous Mater. 2020, 293, 109821–109828. [Google Scholar] [CrossRef]
- Shao, Y.C.; Wang, Y.C.; Liu, X.F.; Li, T.D.; Haydel, P.R.; Tatsumi, T.; Wang, J.G. A single-crystalline hierarchical zeolite via an oriented co-growth of nanocrystals based on synergy of polyelectrolytes and hetero-atoms. ChemCatChem 2020, 12, 2702–2707. [Google Scholar] [CrossRef]
- Tian, Q.W.; Liu, Z.H.; Zhu, Y.H.; Dong, X.L.; Saih, Y.; Basset, J.M.; Sun, M.; Xu, W.; Zhu, L.K.; Zhang, D.L.; et al. Beyond creation of mesoporosity: The advantages of polymer-based dual-function templates for fabricating hierarchical zeolites. Adv. Funct. Mater. 2016, 26, 1881–1891. [Google Scholar] [CrossRef]
- Shen, X.F.; Mao, W.T.; Ma, Y.H.; Xu, D.D.; Wu, P.; Terasaki, O.; Han, L.; Che, S.A.A. A hierarchical MFI zeolite with a two-dimensional square mesostructure. Angew. Chem. Int. Ed. 2018, 57, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Stöcker, M.; Schmidt, R. Composites of micro- and mesoporous materials: Simultaneous syntheses of MFI/MCM-41 like phases by a mixed template approach. Microporous Mesoporous Mater. 1999, 27, 181–192. [Google Scholar] [CrossRef]
- Huang, L.; Guo, W.; Deng, P.; Xue, Z.; Li, Q. Investigation of synthesizing MCM-41/ZSM-5 composites. J. Phys. Chem. B 2000, 104, 2817–2823. [Google Scholar] [CrossRef]
- Petkov, N.; Hölzl, M.; Metzger, T.; Mintova, S.; Bein, T. Ordered micro/mesoporous composite prepared as thin films. J. Phys. Chem. B 2005, 109, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Yilmaz, B.; Müller, U.; Bein, T. Hierarchical Beta zeolite via nanoparticle assembly with a cationic polymer. Chem. Mater. 2011, 23, 4301–4310. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Rodrıguez, J.M. Zeolite beta with hierarchical porosity prepared from organofunctionalized seeds. Microporous Mesoporous Mater. 2008, 115, 504–513. [Google Scholar] [CrossRef]
- Caldeira, V.P.S.; Peral, A.; Linares, M.; Araujo, A.S. Properties of hierarchical Beta zeolites prepared from protozeolitic nanounits for the catalytic cracking of high density polyethylene. Appl. Catal. A Gen. 2017, 531, 187–196. [Google Scholar] [CrossRef]
- Castro, M.; Losch, P.; Park, W.; Haouas, M.; Taulelle, F. Unraveling direct formation of hierarchical Beta zeolite by dynamic light scattering, small angle X-ray scattering, and liquid and solidstate NMR: Insights at the supramolecular level. Chem. Mater. 2018, 30, 2676–2686. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.W.; Yan, X.; Xi, H.X. In Situ Assembly of nanoparticles into hierarchical Beta zeolite with tailored simple organic molecule. Langmuir 2017, 33, 14396–14404. [Google Scholar] [CrossRef]
- Liu, L.J.; Wang, H.B.; Wang, R.W.; Zhang, Z.T. Hydrothermal synthesis of single-crystalline mesoporous Beta zeolite assisted by N-methyl-2-pyrrolidone. RSC Adv. 2014, 4, 39297. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef]
- Naik, S.; Chiang, A.; Thompson, R.; Huang, F. Formation of silicalite-1 hollow spheres by the self-assembly of nanocrystals. Chem. Mater. 2003, 15, 787–792. [Google Scholar] [CrossRef]
- Holland, B.T. Transformation of mostly amorphous mesoscopic aluminosilicate colloids into high surface area mesoporous ZSM-5. Microporous Mesoporous Mater. 2006, 89, 291–299. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Cho, H.J.; Kung, S.C.; Snyder, M.A.; Fan, W. Direct, single-step synthesis of hierarchical zeolites without secondary templating. J. Mater. Chem. A 2015, 3, 1298–1305. [Google Scholar] [CrossRef]
- Xiong, G.; Feng, M.M.; Liu, J.X.; Meng, Q.R.; Liu, L.P.; Guo, H.C. The synthesis of hierarchical high-silica Beta zeolites in NaF media. RSC Adv. 2019, 9, 3653. [Google Scholar] [CrossRef]
- Zhao, X.B.; Wang, L.Y.; Guo, P.; Liu, Z.M. Synthesis of high Si hierarchical Beta zeolites without mesoporogen and their catalytic application in the methanol to propene reaction. Catal. Sci. Technol. 2018, 8, 2966–2974. [Google Scholar] [CrossRef]










| Synthesis Method | Characteristics | Advantages/Disadvantages | Refs. |
|---|---|---|---|
| Template-assisted | Synthesis of certain zeolite framework structure with template assistance | Simplicity and obtaining desired homogenous zeolitic phase nanoparticles/Environmental and economic issues in accordance to SDA and time consumption | [41,42,43,44,45] |
| Seed-assisted | Adding small amount of seeds to synthetic gel without template | Desired zeolitic phase with high yield/Seed introduction | [46,47,48,49,50,51,52,53] |
| Steam-assisted | Crystallization of dry gel containing organic template under water-steaming treatment | Efficient collection of nanosized zeolite and template utilization/Not suitable for operation, and not used in mass production | [54,55] |
| Synthesis Method | Characteristics | Advantages/Disadvantages | Refs. |
|---|---|---|---|
| Dealumination | Introducing mesopores by selectively removing aluminum atoms from the zeolite framework | The process is simple and easy to industrialization/Decreasing the crystallinity of the zeolite | [59,60,61,62,63,64] |
| Desilication | Introducing mesopores by selectively removing silica atoms from the zeolite framework | Simple technique and low cost/Reduces the number of zeolite acid centers and the pores are not connected | [65,66,67,68,69,70,71,72] |
| Dealumination-desilication | Combination of desilication and dealumination methods | Controllable pore structure/The process is complex | [73,74] |
| Hard template | Adding small amount of hard template into the synthetic gel | Wide secondary pore size distribution/Poor thermal stability and mechanical stability | [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] |
| Soft template method | Adding small amount of soft template into the synthetic gel | Good compatibility with precursor of zeolite, pore size distribution is adjustable/Templates are expensive, process is complex | [94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] |
| Template-free method | Using the microporous template agent | Rich in intragranular mesopores and low cost/Poor thermal and mechanical stability | [111,112,113,114,115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.; Zang, J.; Li, B.; Liu, G.; Wang, Y.; Wu, L. Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites. Inorganics 2023, 11, 214. https://doi.org/10.3390/inorganics11050214
Hong L, Zang J, Li B, Liu G, Wang Y, Wu L. Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites. Inorganics. 2023; 11(5):214. https://doi.org/10.3390/inorganics11050214
Chicago/Turabian StyleHong, Luwei, Jiazhong Zang, Bin Li, Guanfeng Liu, Yinbin Wang, and Luming Wu. 2023. "Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites" Inorganics 11, no. 5: 214. https://doi.org/10.3390/inorganics11050214
APA StyleHong, L., Zang, J., Li, B., Liu, G., Wang, Y., & Wu, L. (2023). Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites. Inorganics, 11(5), 214. https://doi.org/10.3390/inorganics11050214