Two Isomers of a Novel Ag(I) Complex with Pyrazole-Type Ligand—Synthesis, Structural, Thermal, and Antioxidative Characterization
Abstract
:1. Introduction
2. Results and Discussion
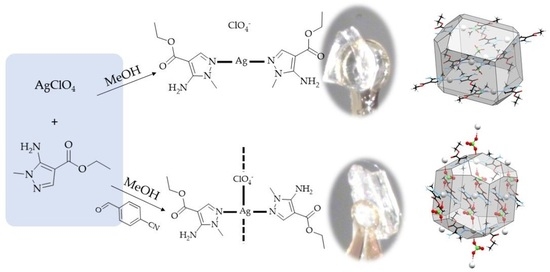
2.1. Compound Syntheses
2.2. Crystal and Molecular Structure
2.3. IR Spectra of L, 1, and 2
2.4. Thermal Properties
2.5. Antioxidative Properties
3. Materials and Methods
3.1. Synthesis of [AgL2]ClO4 (1) with Same-Oriented Pyrazole-Type Ligands
3.2. Synthesis of {[AgL2]ClO4}n (2) with Reverse-Oriented Pyrazole-Type Ligands
3.3. Analytical Techniques
3.4. Crystal Structure Determination
3.5. Determination of DPPH• Radical Neutralization Capability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavrenova, L.G.; Ivanova, A.D.; Bogomyakov, A.S.; Smolentsev, A.I.; Burdukov, A.B.; Sheludyakova, L.A.; Vasilevskii, S.F. Cobalt(II), Nickel(II), and Copper(II) Bromide Complexes with 3-Amino-4-Ethoxycarbonylpyrazole: Syntheses, Structures, and Magnetic Properties. Russ. J. Coord. Chem./Koord. Khimiya 2015, 41, 86–92. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Korotaev, E.V.; Petrov, S.A.; Varnek, V.A.; Lavrenova, L.G. Spin Crossover in Iron(II) Complexes with Tris(Pyrazol-1-Yl)Methane and [Ag(CN)2]- and [Au(CN)2]- Anions. J. Struct. Chem. 2022, 63, 1538–1550. [Google Scholar] [CrossRef]
- Berezin, A.S.; Komarovskikh, A.Y.; Komarov, V.Y.; Syrokvashin, M.M.; Sheven, D.G. Trinuclear Copper(II) Bromide Complex [C3H5N3Br]2: N[Cu3Br8]n. Structure, Magnetic Properties and DFT Calculations. New J. Chem. 2019, 43, 18203–18209. [Google Scholar] [CrossRef]
- Berezin, A.S.; Nadolinny, V.A.; Lavrenova, L.G. Synthesis, Structure, and Properties of a Copper Bromide Coordination Compound with 3-Amino-4-EthoxyCarbonylPyrazole. Nature of the Nonresonant and Ferromagnetic Absorption Observed by EPR. J. Supercond Nov. Magn. 2015, 28, 1007–1011. [Google Scholar] [CrossRef]
- Berezin, A.S.; Ivanova, A.D.; Komarov, V.Y.; Nadolinny, V.A.; Lavrenova, L.G. Influence of Water on the Structure and Magnetic Properties of a Copper Bromide Coordination Compound with 3-Amino-4-Ethoxycarbonylpyrazole. New J. Chem. 2018, 42, 4902–4908. [Google Scholar] [CrossRef]
- Netto, A.V.G.; Frem, R.C.G.; Mauro, A.E.; Crespi, M.S.; Zorel, H.E. Synthesis, Spectral and Thermal Studies on Pyrazolate-Bridged Palladium(II) Coordination Polymers. J. Therm. Anal. Calorim. 2007, 87, 789–792. [Google Scholar] [CrossRef]
- Matiadis, D.; Karagiaouri, M.; Mavroidi, B.; Nowak, K.E.; Katsipis, G.; Pelecanou, M.; Pantazaki, A.; Sagnou, M. Synthesis and Antimicrobial Evaluation of a Pyrazoline-Pyridine Silver(I) Complex: DNA-Interaction and Anti-Biofilm Activity. BioMetals 2021, 34, 67–85. [Google Scholar] [CrossRef]
- Patil, P.; Yadav, A.; Bavkar, L.; Nippu, B.N.; Satyanarayan, N.D.; Mane, A.; Gurav, A.; Hangirgekar, S.; Sankpal, S. [MerDABCO-SO 3 H]Cl Catalyzed Synthesis, Antimicrobial and Antioxidant Evaluation and Molecular Docking Study of Pyrazolopyranopyrimidines. J. Mol. Struct. 2021, 1242, 130672. [Google Scholar] [CrossRef]
- Soliman, S.M.; Almarhoon, Z.; Sholkamy, E.N.; El-Faham, A. Bis-Pyrazolyl-s-Triazine Ni(II) Pincer Complexes as Selective Gram Positive Antibacterial Agents; Synthesis, Structural and Antimicrobial Studies. J. Mol. Struct. 2019, 1195, 315–322. [Google Scholar] [CrossRef]
- Badaweya, E.-S.A.M.; El-Ashmaweyb, I.M. Nonsteroidal Antiinflammatory Agents-Part 1: Antiinflammatory, Analgesic and Antipyretic Activity of Some New 1-(Pyrimidin-2-Yl)-3-Pyrazolin-5ones and 2-(Pyrimidin-2-Yl)-l,2,4,5,6,7-Hexahydro-3H-Indazol-3-Ones. Eur. J. Med. Chem. 1998, 33, 349–361. [Google Scholar] [CrossRef]
- Gökhan-Kelekçi, N.; Yabanoǧlu, S.; Küpeli, E.; Salgin, U.; Özgen, Ö.; Uçar, G.; Yeşilada, E.; Kendi, E.; Yeşilada, A.; Bilgin, A.A. A New Therapeutic Approach in Alzheimer Disease: Some Novel Pyrazole Derivatives as Dual MAO-B Inhibitors and Antiinflammatory Analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786. [Google Scholar] [CrossRef] [PubMed]
- Barra, C.V.; Rocha, F.V.; Netto, A.V.G.; Frem, R.C.G.; Mauro, A.E.; Carlos, I.Z.; Ananias, S.R.; Quilles, M.B. New Palladium(II) Complexes with Pyrazole Ligands: Part II. Synthesis, Spectral and Thermal Studies, and Antitumor Evaluation. J. Therm. Anal. Calorim. 2011, 106, 483–488. [Google Scholar] [CrossRef]
- de Andrade Querino, A.L.; Enes, K.B.; Chaves, O.A.; Dittz, D.; Couri, M.R.C.; Diniz, R.; Silva, H. Modified Pyrazole Platinum(II) Complex Can Circumvent Albumin and Glutathione: Synthesis, Structure and Cytotoxic Activity. Bioorg Chem. 2020, 100, 103936. [Google Scholar] [CrossRef]
- Santos, A.F.; Ferreira, I.P.; Pinheiro, C.B.; Takahashi, J.A.; Teixeira, L.R.; Beraldo, H. Silver(I) Complexes with 2,6-Diacetylpyridine-Bis(Benzoylhydrazones): Antifungal Activity and Interaction with DNA. Polyhedron 2017, 138, 270–276. [Google Scholar] [CrossRef]
- Ristić, P.; Filipović, N.; Blagojević, V.; Ćirković, J.; Barta Holló, B.; Đokić, V.R.; Donnard, M.; Gulea, M.; Marjanović, I.; Klisurić, O.R.; et al. 2D and 3D Silver-Based Coordination Polymers with Thiomorpholine-4-Carbonitrile and Piperazine-1,4-Dicarbonitrile: Structure, Intermolecular Interactions, Photocatalysis, and Thermal Behavior. CrystEngComm 2021, 23, 4799–4815. [Google Scholar] [CrossRef]
- Ristić, P.; Todorović, T.R.; Blagojević, V.; Klisurić, O.R.; Marjanović, I.; Barta Holló, B.; Vulić, P.; Gulea, M.; Donnard, M.; Monge, M.; et al. 1D and 2D Silver-Based Coordination Polymers with Thiomorpholine-4-Carbonitrile and Aromatic Polyoxoacids as Coligands: Structure, Photocatalysis, Photoluminescence, and TD-DFT Study. Cryst. Growth Des. 2020, 20, 4461–4478. [Google Scholar] [CrossRef]
- Barta Holló, B.; Vojinović Ješić, L.S.; Radanović, M.M.; Rodić, M.V.; Jaćimović, Ž.K.; Mészáros Szécsényi, K. Synthesis, Physicochemical, and Thermal Characterization of Coordination Compounds of Cu(II) with a Pyrazole-Type Ligand. J. Therm. Anal. Calorim. 2020, 142, 451–460. [Google Scholar] [CrossRef]
- Barta Holló, B.; Radanović, M.M.; Rodić, M.V.; Krstić, S.; Jaćimović, Ž.K.; Vojinović Ješić, L.S. Synthesis, Physicochemical, Thermal and Antioxidative Properties of Zn(II) Coordination Compounds with Pyrazole-Type Ligand. Inorganics 2022, 10, 20. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Guo, Q.; Englert, U. An Acetylacetonate or a Pyrazole? Both! 3-(3,5-Dimethyl-Pyrazol-4-Yl)Pentane-2,4-Dione as a Ditopic Ligand. Cryst. Growth Des. 2016, 16, 5127–5135. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Akinremi, C.A.; Adewuyi, S.; Lawal, A.; Bamigboye, M.O.; Obaleye, J.A. Some Nontoxic Metal-Based Drugs for Selected Prevalent Tropical Pathogenic Diseases. J. Biol. Inorg. Chem. 2017, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Hofer, A.K.; Edler, K.L.; Ferrence, G.M. Bis(3,5-Dimethyl-1H-Pyrazole-KN2)Silver(I) Hexafluoridoantimonate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m496. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Ning, Y.; Yang, Y.; Hou, X.; Ying, J.; Liu, G.; Zhang, J.; Wang, X. Three New POM-Based Compounds Constructed by Rigid Thiabendazole and Flexible Bis(Pyrazole) Ligands: Structures and Properties for Hg2+ Recognition. Dalton Trans. 2015, 44, 16486–16493. [Google Scholar] [CrossRef] [PubMed]
- Garai, M.; Biradha, K. Coordination Polymers of Organic Polymers Synthesized via Photopolymerization of Single Crystals: Two-Dimensional Hydrogen Bonding Layers with Amazing Shock Absorbing Nature. Chem. Commun. 2014, 50, 3568–3570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Deng, Z.P.; Huo, L.H.; Zhao, H.; Gao, S. Anion-Assisted Silver(I) Coordination Complexes from Flexible Unsymmetrical Bis(Pyridyl) Ligands: Syntheses, Structures and Luminescent Properties. Polyhedron 2013, 59, 38–47. [Google Scholar] [CrossRef]
- May, N.V.; Bayat, N.; Béres, K.A.; Bombicz, P.; Petruševski, V.M.; Lendvay, G.; Farkas, A.; Kótai, L. Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)Silver(I) Perchlorate, [Agpy2ClO4]·0.5py. Inorganics 2022, 10, 123. [Google Scholar] [CrossRef]
- Lissner, F.; Hartenbach, I.; Schleid, T. K3Er7S12 Und Rb3Er7S12: Zwei Ternäre Erbium(III)-Sulfide Mit Kanalstruktur. Z Anorg. Allg. Chem. 2002, 628, 1552–1555. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Béres, K.A.; Kótai, L. Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed Agxo4 (x = Cl, Mn, Re) Compounds. Inorganics 2021, 9, 79. [Google Scholar] [CrossRef]
- Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. [Google Scholar] [CrossRef]
- Lewis, D.L.; Estes, E.D.; Hodgson, D.J. The Infrared Spectra of Coordinated Perchlorates. J. Cryst. Mol. Struct. 1975, 5, 67–74. [Google Scholar] [CrossRef]
- Greenwood, Ν.N.; Earnshaw, A. Chemistry of the Elements; Elsevier B.; Elsevier Butterworth-Heinemann: Oxford, UK, 1997; ISBN 0 7506 3365 4. [Google Scholar]
- Welcome to the NIST WebBook. Available online: https://webbook.nist.gov/ (accessed on 1 October 2021).
- Rigaku Oxford. Diffraction CrysAlisPro Software System; Rigaku Corporation: Wroclaw, Poland, 2019. [Google Scholar]
- Sheldrick, G.M. Foundations and Advances SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallographica Section A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Momerwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An Easy and Fast Test to Compare Total Free Radical Scavenger Capacity of Foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]









| Bond | Distances, Å | Angles, ° | Symmetry Operation on A | ||
|---|---|---|---|---|---|
| D–H···A | D–H | H···A | D···A | D–H···A | |
| 1 | |||||
| N3A–H3AB···O1B(i) | 0.84(2) | 2.24(2) | 3.019(3) | 163 (3) | −x + 3/2, y − 1/2, −z + 3/2 |
| N3B–H3BB···O1A(ii) | 0.84(2) | 2.20(2) | 2.990(3) | 170 (3) | −x + 3/2, y − 1/2, −z + 3/2 |
| N3A–H3AA···O4C(iii) | 0.84(2) | 2.43(2) | 3.247(8) | 126 (3) | |
| N3B–H3BA···O3C(iv) | 0.81(2) | 2.18(2) | 2.963(6) | 155 (3) | x + 3/2, −y + 1/2, z + 1/2 |
| N3A–H3AB···O1A | 0.84(2) | 2.39(3) | 2.956(3) | 162 (4) | x − 1/2, −y + 1/2, z − 1/2 |
| N3B–H3BB···O1B | 0.84(2) | 2.36(3) | 2.894(3) | 157 (3) | x − 3/2, −y + 1/2, z − 1/2 |
| N3A–H3AA···O1D(v) | 0.84(2) | 2.34(2) | 3.170(12) | 123 (3) | |
| 2 | 167 (4) | −x + 1, y−1, −z + 3/2 | |||
| N3–H3B···O1(v) | 0.84(2) | 2.14(3) | 2.903(3) | 124 (4) | |
| N3–H3A···O2C(vi) | 0.84(2) | 2.28(2) | 3.107(4) | 151 (4) | −x + 1/2, −y − 1/2, −z + 1 |
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C14H22AgClN6O8 | C14H22AgClN6O8 |
| Mr | 545.69 | 545.69 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | C2/c |
| a/Å | 9.96262 (16) | 19.2386 (6) |
| b/Å | 20.2514 (4) | 7.3795 (2) |
| c/Å | 10.5085 (3) | 15.4908 (5) |
| α/° | 90 | 90 |
| β/° | 102.5209 (19) | 93.943 (3) |
| γ/° | 90 | 90 |
| V/Å3 | 2069.74 (7) | 2194.03 (12) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| µ/mm−1 | 1.16 | 1.09 |
| Crystal size, mm | 0.39 × 0.32 × 0.19 | 0.48 × 0.23 × 0.18 |
| Data collection | ||
| Absorption correction | Analytical | Analytical |
| Tmin | 0.724 | 0.685 |
| Tmax | 0.829 | 0.860 |
| Measured reflections | 27,536 | 12,513 |
| Independent reflections | 5183 | 2718 |
| Observed reflections [I > 2σ(I)] | 3761 | 2173 |
| Rint | 0.031 | 0.043 |
| (sin θ/λ)max/Å−1 | 0.628 | 0.625 |
| Refinement | ||
| R [F2 > 2σ(F2)] | 0.036 | 0.039 |
| wR [F2] | 0.096 | 0.103 |
| S | 1.02 | 1.06 |
| Reflections | 5183 | 2718 |
| Parameters | 345 | 148 |
| H-atom treatment | Mixed | Mixed |
| Δρmax/e Å−3 | 0.43 | 0.45 |
| Δρmin/e Å−3 | −0.55 | −0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radnović, N.D.; Štetin, N.; Radanović, M.M.; Borišev, I.Đ.; Rodić, M.V.; Jaćimović, Ž.K.; Barta Holló, B. Two Isomers of a Novel Ag(I) Complex with Pyrazole-Type Ligand—Synthesis, Structural, Thermal, and Antioxidative Characterization. Inorganics 2024, 12, 4. https://doi.org/10.3390/inorganics12010004
Radnović ND, Štetin N, Radanović MM, Borišev IĐ, Rodić MV, Jaćimović ŽK, Barta Holló B. Two Isomers of a Novel Ag(I) Complex with Pyrazole-Type Ligand—Synthesis, Structural, Thermal, and Antioxidative Characterization. Inorganics. 2024; 12(1):4. https://doi.org/10.3390/inorganics12010004
Chicago/Turabian StyleRadnović, Nikola D., Nađa Štetin, Mirjana M. Radanović, Ivana Đ. Borišev, Marko V. Rodić, Željko K. Jaćimović, and Berta Barta Holló. 2024. "Two Isomers of a Novel Ag(I) Complex with Pyrazole-Type Ligand—Synthesis, Structural, Thermal, and Antioxidative Characterization" Inorganics 12, no. 1: 4. https://doi.org/10.3390/inorganics12010004
APA StyleRadnović, N. D., Štetin, N., Radanović, M. M., Borišev, I. Đ., Rodić, M. V., Jaćimović, Ž. K., & Barta Holló, B. (2024). Two Isomers of a Novel Ag(I) Complex with Pyrazole-Type Ligand—Synthesis, Structural, Thermal, and Antioxidative Characterization. Inorganics, 12(1), 4. https://doi.org/10.3390/inorganics12010004