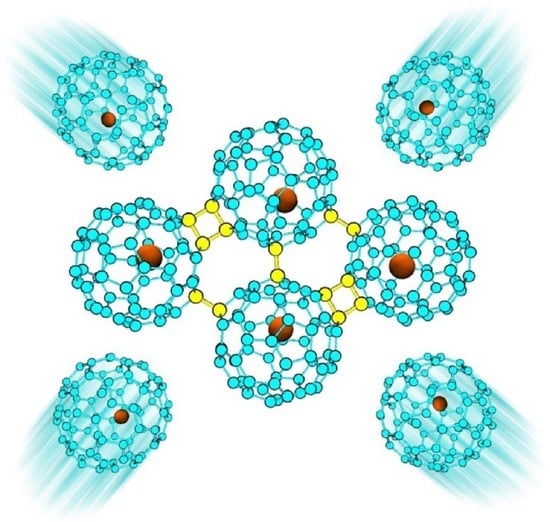
Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers
Abstract
:1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindle, J.; Pong, R.; Bartoli, F.; Kafafi, Z. Nonlinear optical properties of the fullerenes C60 and C70 at 1.064 μm. Phys. Rev. B 1993, 48, 9447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Lawson, G.E.; Riggs, J.E.; Ma, B.; Wang, N.; Moton, D.K. Photophysical and nonlinear optical properties of [60] fullerene derivatives. J. Phys. Chem. A 1998, 102, 5520–5528. [Google Scholar] [CrossRef]
- Makarova, T. Electrical and optical properties of pristine and polymerized fullerenes. Semiconductors 2001, 35, 243–278. [Google Scholar] [CrossRef]
- Sachdeva, S.; Singh, D.; Tripathi, S. Optical and electrical properties of fullerene C70 for solar cell applications. Opt. Mater. 2020, 101, 109717. [Google Scholar] [CrossRef]
- Guo, K.; Li, N.; Bao, L.; Lu, X. Fullerenes and derivatives as electrocatalysts: Promises and challenges. Green Energy Environ. 2022, 9, 7–27. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Y.; Li, M.; Qi, X.; Pan, C.; Guo, K.; Bao, L.; Lu, X. Ultrahigh Capacity from Complexation-Enabled Aluminum-Ion Batteries with C70 as the Cathode. Adv. Mater. 2023, 2306244. [Google Scholar] [CrossRef]
- Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- Collavini, S.; Delgado, J.L. Fullerenes: The stars of photovoltaics. Sustain. Energy Fuels 2018, 2, 2480–2493. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Su, S.; Zhen, M.; Zhou, C.; Cao, X.; Sun, Z.; Xu, Y.; Li, L.; Jia, W.; Wu, Z.; Wang, C. Efficiently Inhibiting Systemic Inflammatory Cascades by Fullerenes for Retarding HFD-Fueled Atherosclerosis. Adv. Healthc. Mater. 2023, 12, 2202161. [Google Scholar] [CrossRef]
- Burger, B.; Winter, J.; Kuzmany, H. Dimer and cluster formation in C60 photoreaction. Z. Phys. B Condens. Matter 1996, 101, 227–233. [Google Scholar] [CrossRef]
- Blank, V.D.; Buga, S.G.; Dubitsky, G.A.; Serebryanaya, N.R.; Popov, M.Y.; Sundqvist, B. High-pressure polymerized phases of C60. Carbon 1998, 36, 319–343. [Google Scholar] [CrossRef]
- Okada, S.; Saito, S. Electronic structure and energetics of pressure-induced two-dimensional C60 polymers. Phys. Rev. B 1999, 59, 1930. [Google Scholar] [CrossRef]
- Xu, C.H.; Scuseria, G.E. Theoretical predictions for a two-dimensional rhombohedral phase of solid C60. Phys. Rev. Lett. 1995, 74, 274. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Eklund, P.; Venkateswaran, U.; Tucker, J.; Duncan, M.; Bendele, G.; Stephens, P.; Hodeau, J.L.; Marques, L.; Nunez-Regueiro, M.; et al. Properties of C60 polymerized under high pressure and temperature. Appl. Phys. A 1997, 64, 231–2239. [Google Scholar] [CrossRef]
- Giacalone, F.; Martin, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Blank, V.; Buga, S.; Serebryanaya, N.; Denisov, V.; Dubitsky, G.; Ivlev, A.; Mavrin, B.; Popov, M.Y. Ultrahard and superhard carbon phases produced from C60 by heating at high pressure: Structural and Raman studies. Phys. Lett. A 1995, 205, 208–216. [Google Scholar] [CrossRef]
- Sabirov, D.S. Polarizability of C60 fullerene dimer and oligomers: The unexpected enhancement and its use for rational design of fullerene-based nanostructures with adjustable properties. RSC Adv. 2013, 3, 19430–19439. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Ori, O.; Tukhbatullina, A.A.; Shepelevich, I.S. Covalently bonded fullerene nano-aggregates (C60)n: Digitalizing their energy–topology–symmetry. Symmetry 2021, 13, 1899. [Google Scholar] [CrossRef]
- Hou, L.; Cui, X.; Guan, B.; Wang, S.; Li, R.; Liu, Y.; Zhu, D.; Zheng, J. Synthesis of a monolayer fullerene network. Nature 2022, 606, 507–510. [Google Scholar] [CrossRef]
- Meirzadeh, E.; Evans, A.M.; Rezaee, M.; Milich, M.; Dionne, C.J.; Darlington, T.P.; Bao, S.T.; Bartholomew, A.K.; Handa, T.; Rizzo, D.J.; et al. A few-layer covalent network of fullerenes. Nature 2023, 613, 71–76. [Google Scholar] [CrossRef]
- Argaman, U.; Makov, G. Structure and properties of graphullerene: A semiconducting two-dimensional C60 crystal. Npj Comput. Mater. 2023, 9, 211. [Google Scholar] [CrossRef]
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843. [Google Scholar] [CrossRef]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Yu, B.; Akasaka, T.; Lu, X. Endohedral metallofullerenes: An unconventional core–shell coordination union. Coord. Chem. Rev. 2013, 257, 2880–2898. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, C. Endohedral Metallofullerenes Based on Spherical Ih-C80 Cage: Molecular Structures and Paramagnetic Properties. Acc. Chem. Res. 2014, 47, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, Y.; Magagula, S.; Chen, Z. Exohedral functionalization of endohedral metallofullerenes: Interplay between inside and outside. Coord. Chem. Rev. 2019, 388, 406–439. [Google Scholar] [CrossRef]
- Shen, W.; Hu, S.; Lu, X. Endohedral metallofullerenes: New structures and unseen phenomena. Chem.-Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Dang, J.; Zhao, X. Theoretical study on the stabilities, electronic structures, and reaction and formation mechanisms of fullerenes and endohedral metallofullerenes. Coord. Chem. Rev. 2022, 471, 214762. [Google Scholar] [CrossRef]
- Shen, W.; Bao, L.; Lu, X. Endohedral Metallofullerenes: An Ideal Platform of Sub-Nano Chemistry. Chin. J. Chem. 2022, 40, 275–284. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Ullah, A.; Gutiérrez-Finol, G.M.; Bedoya-Pinto, A.; Gargiani, P.; Shi, D.; Yang, S.; Shi, Z.; Gaita-Ariño, A.; et al. High-temperature magnetic blocking in a monometallic dysprosium azafullerene single-molecule magnet. Chem 2023, 9, 3613–3622. [Google Scholar] [CrossRef]
- Kubozono, Y.; Noto, T.; Ohta, T.; Maeda, H.; Kashino, S.; Emura, S.; Ukita, S.; Sogabe, T. Extractions of Ca@C60 and Sr@C60 with aniline. Chem. Lett. 1996, 25, 453–454. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Deng, J.; Wang, Z. Capturing unconventional metallofullerene M@C60 through activation of the unreactive [5,6] bond toward Diels–Alder reaction. Phys. Chem. Chem. Phys. 2020, 22, 24249–24256. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Aoyagi, S.; Yamazaki, Y.; Ohkubo, K.; Ikuma, N.; Okada, H.; Kato, T.; Matsuo, Y.; Fukuzumi, S.; Kokubo, K. Electrochemical reduction of cationic Li+@C60 to neutral Li+@C60˙−: Isolation and characterisation of endohedral [60]fulleride. Chem. Sci. 2016, 7, 5770–5774. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hamann, D.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef]









| HOMO-LUMO Gap | |
|---|---|
| Ca@C | 1.14 |
| 2-1 | 0.50 |
| 2-2 | 1.07 |
| 2-3 | 1.36 |
| 2-4 | 0.94 |
| 3-1 | 0.22 |
| 3-2 | 1.02 |
| 3-3 | 1.21 |
| 3-4 | 0.79 |
| 3-5 | 0.60 |
| 4-1 | 0.14 |
| 4-2 | 1.05 |
| 4-3 | 1.14 |
| 4-4 | 0.80 |
| 4-5 | 0.38 |
| 4-6 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhou, Z.; Wang, Z. Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics 2024, 12, 45. https://doi.org/10.3390/inorganics12020045
Wu Y, Zhou Z, Wang Z. Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics. 2024; 12(2):45. https://doi.org/10.3390/inorganics12020045
Chicago/Turabian StyleWu, Yabei, Zhonghao Zhou, and Zhiyong Wang. 2024. "Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers" Inorganics 12, no. 2: 45. https://doi.org/10.3390/inorganics12020045
APA StyleWu, Y., Zhou, Z., & Wang, Z. (2024). Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics, 12(2), 45. https://doi.org/10.3390/inorganics12020045