Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
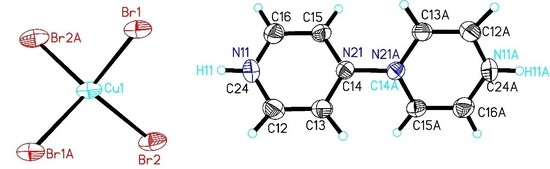
2.2. Structure
2.3. Magnetism
2.4. Powder X-Band EPR
3. Materials and Methods
3.1. Synthesis
3.2. Magnetism
3.3. Crystal Structure Data Collection, Solution and Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bu, R.; Xiong, Y.; Wei, X.; Li, H.; Zhang, C. Hydrogen Bonding in CHON-Containing Energetic Crystals: A Review. Cryst. Growth Des. 2019, 19, 5981–5997. [Google Scholar] [CrossRef]
- Saha, S.; Mishra, M.K.; Reddy, C.M.; Desiraju, G.R. From Molecules to Interactions to Crystal Engineering: Mechanical Properties of Organic Solids. Acc. Chem. Res. 2018, 51, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Grepioni, F.; Maini, L.; d’Agostino, S. From Solid-State Structure and Dynamics to Crystal Engineering. Eur. J. Inorg. Chem. 2018, 2018, 3597–3605. [Google Scholar] [CrossRef]
- Lago, A.B.N.; Carballo, R.; Garciía-Martiínez, E.; Vaázquez-Loópez, E.M. Metal to Ligand Interactions and Hydrogen Bonding in the Design of Metallosupramolecular Compounds: Effect of pH and Aprotic Solvents on the Nature of Materials Based on Bis(4-pyridylthio)methane and Copper(II) Chloride. Cryst. Growth Des. 2011, 11, 59–68. [Google Scholar] [CrossRef]
- Singh, Y.P.; Patel, R.N.; Singh, Y.; Choquesillo-Lazarte, D.; Butcher, R.J. Classical hydrogen bonding and stacking of chelate rings in new copper(II) complexes. Dalton Trans. 2017, 46, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, A.; Ishida, T. Super–superexchange coupling through a hydrogen bond in a linear copper(II) complex, [Cu(LH)(L)]·BF4·2H2O (LH = N-tert-butyl-N-2-pyridylhydroxylamine). Chem. Phys. Lett. 2009, 480, 198–202. [Google Scholar] [CrossRef]
- Manson, J.L.; Schlueter, J.A.; Funk, K.A.; Southerland, H.I.; Twamley, B.; Lancaster, T.; Blundell, S.J.; Baker, P.J.; Pratt, F.L.; Singleton, J.; et al. Strong H···F Hydrogen Bonds as Synthons in Polymeric Quantum Magnets: Structural, Magnetic, and Theoretical Characterization of [Cu(HF2)(pyrazine)2]SbF6, [Cu2F(HF)(HF2)(pyrazine)](SbF6)2, and [CuAg(H3F4)(pyrazine)5](SbF6)2. J. Am. Chem. Soc. 2009, 131, 6733–6747. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Golobic, A.; Kozlevcar, B.; Robertazzi, A.; Vargiu, A.V.; Gamez, P.; Reedijk, J. Magnetic coupling between copper(II) ions mediated by hydrogen-bonded (neutral) water molecules. Inorg. Chem. 2009, 48, 5473–5479. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Grachvogel, D.A.; Williams, D.J. Combination of weak covalent, hydrogen bonding, and Ag⋯π interactions in the formation of a 3D porous network Ag(pypd)2(ClO4)·CH2Cl2 by the ambidentate ligand 1-(4′-pyridyl)pyridin-4-one. J. Chem. Soc. Dalton Trans. 2002, 11, 2259–2260. [Google Scholar] [CrossRef]
- Kumar, D.K.; Ballabh, A.; Jose, D.A.; Dastidar, P.; Das, A. How Robust Is the N–H···Cl2-Cu Synthon? Crystal Structures of Some Perchlorocuprates. Crys. Growth Des. 2005, 5, 651–660. [Google Scholar]
- Willett, R.D.; Butcher, R.E.; Landee, C.P.; Twamley, B. Two halide exchange in copper(II) halide dimers: (4,4′-Bipyridinium)Cu2Cl6−xBrx. Polyhedron 2006, 25, 2093–2100. [Google Scholar] [CrossRef]
- Yahsi, Y.; Gungor, E.; Kara, H. Chlorometallate-Pyridinium Boronic Acid Salts for Crystal Engineering: Synthesis of One-, Two-, and Three-Dimensional Hydrogen Bond Networks. Cryst. Growth Des. 2015, 15, 2652–2660. [Google Scholar] [CrossRef]
- Dgachi, S.; Ben Salah, A.M.; Turnbull, M.M.; Bataille, T.; Naili, H. Investigations on (C6H9N2)2[MIIBr4] halogenometallate complexes with MII = Co, Cu and Zn: Crystal structure, thermal behavior and magnetic properties. J. Alloys Comps. 2017, 726, 315–322. [Google Scholar] [CrossRef]
- Solomon, B.L.; Landee, C.P.; Turnbull, M.M.; Wikaira, J.L. Copper(II) complexes of 2-amino-5-chloro-3-fluoropyridine: Syntheses and magnetic properties of (3,5-FCAP)2CuX2 and (3,5-FCAPH)2CuX4. J. Coord. Chem. 2014, 67, 3953–3971. [Google Scholar] [CrossRef]
- Hong, T.; Gvasaliya, S.N.; Herringer, S.N.; Turnbull, M.M.; Landee, C.P.; Regnault, L.-P.; Boehm, M.; Zheludev, A. Dynamics of the two-dimensional S = 1/2 dimer system (C5H6N2F)2CuCl4. Phy. Rev. B 2011, 83, 052401. [Google Scholar] [CrossRef] [Green Version]
- Tremelling, G.W.; Foxman, B.M.; Landee, C.P.; Turnbull, M.M.; Willett, R.D. Transition metal complexes of 2-amino-3,5-dihalopyridines: Syntheses, structures and magnetic properties of (3,5-diCAPH)2CuX4 and (3,5-diBAPH)2CuX4. Dalton Trans. 2009, 47, 10518–10526. [Google Scholar] [CrossRef]
- Deumal, M.; Giorgi, G.; Robb, M.A.; Turnbull, M.M.; Landee, C.P.; Novoa, J.J. The mechanism of magnetic interaction in spin-ladder molecular magnets: A first-principles, bottom-up, theoretical study of the magnetism in the two-legged spin-ladder bis(2-amino-5-nitropyridinium) tetrabromocuprate monohydrate. Eur. J. Inorg. Chem. 2005, 2005, 4697–4706. [Google Scholar] [CrossRef]
- Woodward, F.M.; Albrecht, A.S.; Wynn, C.M.; Landee, C.P.; Turnbull, M.M. Two-dimensional S = 1/2 Heisenberg antiferromagnets: Synthesis, structure, and magnetic properties. Phys. Rev. B 2002, 65, 144412. [Google Scholar] [CrossRef] [Green Version]
- Al Damen, M.A.; Haddad, S.F. The nonclassical noncovalent interactions control: A case study of the crystal structure of 3,5-dibromo-2-amino-4,6-dimethylpyridinium tetrahalocuprate [3,5-DBr-2-A-4,6-DMPH]2CuX4 (X = Cl, and Br). J. Mol. Struct. 2011, 985, 27–33. [Google Scholar] [CrossRef]
- Gong, J.; Chen, G.; Ni, S.F.; Zhang, Y.Y.; Wang, H.B. Bis(2-amino-6-methylpyridinium) tetrachloridocuprate(II). Acta Crystallogr. Sect. E 2009, 65, m1661–m1662. [Google Scholar] [CrossRef] [Green Version]
- Kovalchukova, O.V.; Palkina, K.K.; Strashnova, S.B.; Zaitsev, B.E. Synthesis, structure, geometrical, and spectral characteristics of the (HLn)2[CuCl4] complexes. Crystal and molecular structure of bis(2-methylimidazolium) tetrachlorocuprate(II). Russ. J. Coord. Chem. 2008, 34, 830–835. [Google Scholar] [CrossRef]
- Haddad, S.F.; AlDamen, M.A.; Willett, R.D. The role of non-classical supramolecular interactions in the structures of 2-amino-4,6-dimethylpyridinium tetrahalocuprate(II) salts. Inorg. Chim. Acta 2006, 359, 424–432. [Google Scholar] [CrossRef]
- Boutchard, C.L.; Hitchman, M.A.; Skelton, B.W.; White, A.H. Crystal structure and electronic spectrum of the distorted tetrahedral CuCl42− ion in bis(2-aminopyridinium) tetrachlorocuprate(II) and comparison with the tetragonally elongated octahedral complex bis(2-aminopyrimidinium)tetrachlorocopper(II). Aust. J. Chem. 1995, 48, 771–781. [Google Scholar] [CrossRef]
- Place, H.; Willett, R.D. Structure of catalytically related species involving copper(II) halides. III. 2-Amino-5-bromo-3-methylpyridinium 2-amino-3-methylpyridinium tetrabromocuprate(II). Acta Crystallogr. Sect. C 1987, 43, 1497–1500. [Google Scholar] [CrossRef] [Green Version]
- Walha, S.; Naili, H.; Yahyaoui, S.; Ali, B.F.; Turnbull, M.M.; Mhiri, T.; Ng, S.W. Three-Dimensional Network Polymeric Structure of (2-Amino-5-ammoniopyridinium)[CuCl4]: Crystal Supramolecularity and Magnetic Properties. J. Supercond. Nov. Magn. 2013, 26, 437–442. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Park, S.-M.; Kang, S.K.; Kim, Y.-I.; Choi, S.-N. Crystal Structures and Magnetic Properties of Sparteinium Tetrahalocuprate Monohydrate Compounds. Bull. Kor. Chem. Soc. 2004, 25, 823–828. [Google Scholar]
- Wikaira, J.L.; Butcher, R.J.; Kersen, Ü.; Turnbull, M.M. Magnetic properties of 4,4′-bipiperidinediium tetrahalocuprates: Crystal structures of 4,4′-bipiperidinediium tetrabromocuprate(II) monohydrate and bis(4,4′-bipiperidinediium) hexabromodicuprate(I). J. Coord. Chem. 2015, 69, 57–71. [Google Scholar] [CrossRef]
- Turnbull, M.M.; Landee, C.P.; Wells, B.M. Magnetic exchange interactions in tetrabromocuprate compounds. Coord. Chem. Rev. 2005, 249, 2567–2576. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Brenčič, J.V.; Čeh, B.; Leban, I. Structure of 1-(4-pyridyl)pyridinium trans-tetrachlorodi(pyridine)molybdate(III). Acta Crystallogr. Sect. C 1989, 45, 1144–1146. [Google Scholar] [CrossRef]
- Brenčič, J.V.; Čeh, B.; Leban, I. Preparation, Chemical, and Structural Characterization of Some Molybdenum(III) and Tungsten(III) Coordination Compounds Containing 1-(4-pyridyl)pyridinium Cation. Z. Anorg. Allg. Chem. 1988, 565, 163–170. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Grachvogel, D.A.; White, A.J.P.; Williams, D.J. Diverse polymeric metal complexes formed by the ambidentate ligand 1-(4′-pyridyl)pyridin-4-one. Inorg. Chim. Acta 2003, 348, 187–193. [Google Scholar] [CrossRef]
- Angeloni, A.; Crawford, P.C.; Orpen, A.G.; Podesta, T.J.; Shore, B.J. Does Hydrogen Bonding Matter in Crystal Engineering? Crystal Structures of Salts of Isomeric Ions. Chem. Eur. J. 2004, 10, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Gorelsky, S.I.; Ilyukhin, A.B.; Kholin, P.V.; Kotov, V.Y.; Lokshin, B.V.; Sapoletova, N.V. Dihydrohexacyanoferrates of N-heterocyclic cations. Inorg. Chim. Acta 2007, 360, 2573–2582. [Google Scholar] [CrossRef]
- Li, L.; Turnbull, M.M.; Landee, C.P.; Jornet, J.; Deumal, M.; Novoa, J.J.; Wikaira, J.L. Synthesis, structure and magnetic behavior of bis(2-amino-5-fluoropyridinium) tetrachlorocuprate(II). Inorg. Chem. 2007, 46, 11254–11265. [Google Scholar] [CrossRef]
- Landee, C.P.; Turnbull, M.M. A gentle introduction to magnetism: Units, fields, theory, and experiment. J. Coord. Chem. 2014, 67, 375–439. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Farra, R.; Thiel, K.; Winter, A.; Klamroth, T.; Pöppl, A.; Kelling, A.; Schilde, U.; Taubert, A.; Strauch, P. Tetrahalidocuprates(II)—Structure and EPR spectroscopy. Part 1: Tetrabromidocuprates(II). New J. Chem. 2011, 35, 2793. [Google Scholar] [CrossRef] [Green Version]
- Willett, R.D.; Wong, R.J. Experimental Evidence for Phonon Modulation of Antisymmetric Exchange from the Temperature Dependence of EPR Linewidths in (RNH3)2CuX4 Salts. J. Magn. Reson. 1981, 42, 446–452. [Google Scholar] [CrossRef]
- Monroe, J.C.; Rademeyer, M.; Turnbull, M.M. Compounds of pyridylpyridones. In preparation.
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Bruker. SAINT-2012, Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.
- Bruker SADABS. Version 2014/5, Bruker AXS Inc.: Madison, WI, USA.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]








| 1 | 2 | |
|---|---|---|
| Formula | C10H10N2Cl4Cu | C10H10N2Br4Cu |
| Molecular Weight | 363.54 | 541.38 |
| Crystal System | monoclinic | monoclinic |
| Space Group | C2/c | C2/c |
| a(Å) | 15.6790(11) | 16.0672(9) |
| b(Å) | 7.4315(5) | 7.5861(4) |
| c(Å) | 12.0742(8) | 12.5969(6) |
| α(°) | 90 | 90 |
| β(°) | 108.270(3) | 108.126(2) |
| γ(°) | 90 | 90 |
| V(Å3) | 1335.95(16) | 1459.21(13) |
| Z | 4 | 4 |
| T(K) | 150(2) | 150(2) |
| ρcalc (g cm−1) | 1.803 | 2.464 |
| μ(mm−1) | 2.411 | 12.429 |
| λ(Å) | 0.71073 | 0.71073 |
| Index Ranges | −28 ≤ h ≤ 28 | −22 ≤ h ≤ 21 |
| −13 ≤ k ≤ 13 | −10 ≤ k ≤ 10 | |
| −21 ≤ l ≤ 21 | −16 ≤ l ≤ 16 | |
| Indep. Reflections [I > 2σ(I)] | 3060 | 1473 |
| Obs. reflections | 4022 | 1856 |
| Parameters | 94 | 95 |
| Goodness of fit | 1.021 | 1.063 |
| R [I > 2σ(I)] | 0.0432 | 0.0363 |
| Rw [I > 2σ(I)] | 0.0893 | 0.0795 |
| R (all reflections) | 0.0641 | 0.0530 |
| Rw (all reflections) | 0.0983 | 0.0850 |
| 1 | 2 | |
|---|---|---|
| Cu1–X1 | 2.2481(5) | 2.3816(5) |
| Cu1–X2 | 2.2515(5) | 2.3919(5) |
| X1–Cu1–X1a | 140.63(3) | 143.03(4) |
| X2–Cu1–X2a | 145.24(3) | 146.71(4) |
| X1–Cu1–X2 | 95.49(2) | 95.231(19) |
| X1–Cu1–X2a | 96.060(19) | 95.190(18) |
| X1a–Cu1–X2 | 96.060(18) | 95.189(18) |
| X1a–Cu1–X2a | 95.49(2) | 95.230(19) |
| DHA | DH (Å) | H···A (Å) | D···A (Å) | DHA (°) | |
|---|---|---|---|---|---|
| N11–H11···X1 | 1 | 0.91(6) | 2.47(6) | 3.2699(18) | 147(5) |
| 2 | 0.843(14) | 2.66(3) | 3.429(5) | 152(4) | |
| N11–H11···X2 | 1 | 0.91(6) | 2.79(6) | 3.464(2) | 132(4) |
| 2 | 0.843(14) | 2.99(4) | 3.599(5) | 131(4) |
| CC (emu·K/mol·Oe) | θ (K) | gave | R2 | |
|---|---|---|---|---|
| 1 | 0.4406(1) | −0.26(5) | 2.168 | 0.99999 |
| 2 | 0.4157(4) | −1.0(1) | 2.105 | 0.99994 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroe, J.C.; Landee, C.P.; Rademeyer, M.; Turnbull, M.M. Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium. Inorganics 2020, 8, 18. https://doi.org/10.3390/inorganics8030018
Monroe JC, Landee CP, Rademeyer M, Turnbull MM. Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium. Inorganics. 2020; 8(3):18. https://doi.org/10.3390/inorganics8030018
Chicago/Turabian StyleMonroe, Jeffrey C., Christopher P. Landee, Melanie Rademeyer, and Mark M. Turnbull. 2020. "Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium" Inorganics 8, no. 3: 18. https://doi.org/10.3390/inorganics8030018
APA StyleMonroe, J. C., Landee, C. P., Rademeyer, M., & Turnbull, M. M. (2020). Copper(II) Halide Salts with 1-(4′-Pyridyl)-Pyridinediium. Inorganics, 8(3), 18. https://doi.org/10.3390/inorganics8030018