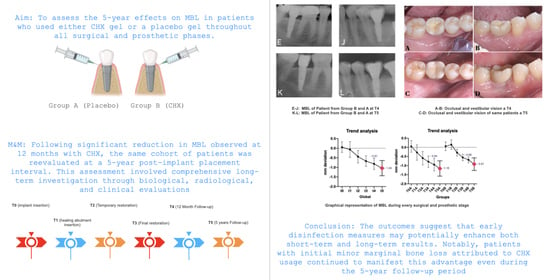
Long-Term Efficacy of Chlorhexidine Gel in Single-Crown Implant Rehabilitation: A Five-Year Follow-Up Study
Abstract
:1. Introduction
- -
- Correlate the MBL described in the first study [23] and evaluate its trend over time.
- -
- Analyze biological complications during 5 years of follow-up.
2. Materials and Methods
2.1. Study Design and Sample
2.2. Sample Size and Randomization
2.3. Surgical and Prosthetic Treatment
2.4. Patient Analysis
- -
- Periapical X-ray;
- -
- FMPS and FMBS;
- -
- Frequency of follow-up visits.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10-16-year follow-up of non-submerged dental implants. Clin. Oral Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- Filius, M.A.P.; Vissink, A.; Cune, M.S.; Raghoebar, G.M.; Visser, A. Long-term implant performance and patients’ satisfaction in oligodontia. J. Dent. 2018, 71, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Baumer, A.; Toekan, S.; Saure, D.; Korner, G. Survival and success of implants in a private periodontal practice: A 10-year retrospective study. BMC Oral Health 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Martins, M.D.; Wennerberg, A. Immediate placement of implants into infected sites: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 1), e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T.; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now? Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Berardini, M.; Trisi, P.; Sinjari, B.; Rutjes, A.W.; Caputi, S. The Effects of High Insertion Torque Versus Low Insertion Torque on Marginal Bone Resorption and Implant Failure Rates: A Systematic Review with Meta-Analyses. Implant Dent. 2016, 25, 532–540. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and esthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 2–21. [Google Scholar] [CrossRef]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef]
- Krisam, J.; Ott, L.; Schmitz, S.; Klotz, A.L.; Seyidaliyeva, A.; Rammelsberg, P.; Zenthöfer, A. Factors affecting the early failure of implants placed in a dental practice with a specialization in implantology—A retrospective study. BMC Oral Health 2019, 19, 208. [Google Scholar] [CrossRef]
- Coli, P.; Christiaens, V.; Sennerby, L.; Bruyn, H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol. 2000 2017, 73, 203–217. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.A.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.S.; Araujo, M.A.; Benfatti, C.A.; Araujo, C.R.; Piattelli, A.; Perrotti, V.; Iezzi, G. Comparative histological and histomorphometrical evaluation of marginal bone resorption around external hexagon and Morse cone implants: An experimental study in dogs. Implant Dent. 2014, 23, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Rivara, F.; Macaluso, G.M.; Toffoli, A.; Calciolari, E.; Goldoni, M.; Lumetti, S. The effect of a 2-mm inter-implant distance on esthetic outcomes in immediately non-occlusally loaded platform shifted implants in healed ridges: 12-month results of a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2020, 22, 486–496. [Google Scholar] [CrossRef]
- Dellow, A.G.; Driessen, C.H.; Nel, H.J. Scanning electron microscopy evaluation of the interfacial fit of interchanged components of four dental implant systems. Int. J. Prosthodont. 1997, 10, 216–221. [Google Scholar]
- D’Ercole, S.; Tripodi, D.; Ravera, L.; Perrotti, V.; Piattelli, A.; Iezzi, G. Bacterial leakage in Morse Cone internal connection implants using different torque values: An in vitro study. Implant Dent. 2014, 23, 175–179. [Google Scholar] [CrossRef]
- Scarano, A.; Perrotti, V.; Piattelli, A.; Iaculli, F.; Iezzi, G. Sealing capability of implant-abutment junction under cyclic loading: A toluidine blue in vitro study. J. Appl. Biomater. Funct. Mater. 2015, 13, e293–e295. [Google Scholar] [CrossRef]
- Ericsson, I.; Persson, L.G.; Berglundh, T.; Marinello, C.P.; Lindhe, J.; Klinge, B. Different types of inflammatory reactions in peri-implant soft tissues. J. Clin. Periodontol. 1995, 22, 255–261. [Google Scholar] [CrossRef]
- Faveri, M.; Gursky, L.C.; Feres, M.; Shibli, J.A.; Salvador, S.L.; de Figueiredo, L.C. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J. Clin. Periodontol. 2006, 33, 819–828. [Google Scholar] [CrossRef]
- Carcuac, O.; Abrahamsson, I.; Charalampakis, G.; Berglundh, T. The effect of the local use of chlorhexidine in surgical treatment of experimental peri-implantitis in dogs. J. Clin. Periodontol. 2015, 42, 196–203. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; van Winkelhoff, A.J. Implant decontamination with 2% chlorhexidine during surgical peri-implantitis treatment: A randomized, double-blind, controlled trial. Clin. Oral Implant. Res. 2015, 26, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennstr√∂m, J.; Berglundh, T. Adjunctive Systemic and Local Antimicrobial Therapy in the Surgical Treatment of Peri-implantitis: A Randomized Controlled Clinical Trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo-A Randomized Double Blind Controlled Human Study. Biomed. Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; D’Addazio, G.; Di Lodovico, S.; Traini, T.; Di Giulio, M.; Sinjari, B. Porphyromonas Gingivalis Load is Balanced by 0.20% Chlorhexidine Gel. A Randomized, Double-Blind, Controlled, Microbiological and Immunohistochemical Human Study. J. Clin. Med. 2020, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Bignozzi, I.; Cristalli, M.P.; Graziani, F.; La Monaca, G.; Polimeni, A. Peri-implant marginal bone level: A systematic review and meta-analysis of studies comparing platform switching versus conventionally restored implants. J. Clin. Periodontol. 2012, 39, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Laurell, L.; Lundgren, D. Marginal bone level changes at dental implants after 5 years in function: A meta-analysis. Clin. Implant Dent. Relat. Res. 2011, 13, 19–28. [Google Scholar] [CrossRef]
- Derks, J.; HaÃäkansson, J.; WennstroÃàm, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and late implant loss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-term effect of surface roughness and patients’ factors on crestal bone loss at dental implants. A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef]
- Zumstein, T.; SchuÃàtz, S.; Sahlin, H.; Sennerby, L. Factors influencing marginal bone loss at a hydrophilic implant design placed with or without GBR procedures: A 5-year retrospective study. Clin. Implant Dent. Relat. Res. 2019, 21, 817–826. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Lin, Z.; Song, Z.; Shu, R.; Xie, Y. Survival rate and potential risk indicators of implant loss in non-smokers and systemically healthy periodontitis patients: An up to 9-year retrospective study. J. Periodont. Res. 2021, 56, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.M.; Geng, W.; Luo, C.C. Prosthetic complications of fixed dental prostheses supported by locking-taper implants: A retrospective study with a mean follow-up of 5 years. BMC Oral Health 2021, 21, 476. [Google Scholar] [CrossRef] [PubMed]
- Gosau, D.M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; BuÃàrgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin. Oral Implant. Res. 2010, 21, 866–872. [Google Scholar] [CrossRef]
- Chin, M.Y.; Sandham, A.; de Vries, J.; van der Mei, H.C.; Busscher, H.J. Biofilm formation on surface characterized micro-implants for skeletal anchorage in orthodontics. Biomaterials 2007, 28, 2032–2040. [Google Scholar] [CrossRef]
- Ntrouka, V.I.; Slot, D.E.; Louropoulou, A.; Van der Weijden, F. The effect of chemotherapeutic agents on contaminated titanium surfaces: A systematic review. Clin. Oral Implant. Res. 2011, 22, 681–690. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; Bozzi, M.; Celletti, R.; Traini, T.; Mavriqi, L.; Caputi, S. Comparison of a Novel Ultrasonic Scaler Tip vs. Conventional Design on a Titanium Surface. Materials 2018, 11, 2345. [Google Scholar] [CrossRef]
- Anitua, E.; Orive, G.; Aguirre, J.J.; Ardanza, B.; Andia, I. 5-year clinical experience with BTI dental implants: Risk factors for implant failure. J. Clin. Periodontol. 2008, 35, 724–732. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Huynh-Ba, G. History of treated periodontitis and smoking as risks for implant therapy. Int. J. Oral Maxillofac. Implant. 2009, 24, 39–68. [Google Scholar]
- Mayta-Tovalino, F.; Mendoza-Martiarena, Y.; Romero-Tapia, P.; Álvarez-Paucar, M.; Gálvez-Calla, L.; Calderón-Sánchez, J.; Bolaños-Cardenas, R.; Diaz-Sarabia, A. An 11-Year Retrospective Research Study of the Predictive Factors of Peri-Implantitis and Implant Failure: Analytic-Multicentric Study of 1279 Implants in Peru. Int. J. Dent. 2019, 2019, 3527872. [Google Scholar] [CrossRef]
- Renvert, S.; Quirynen, M. Risk indicators for peri-implantitis. A narrative review. Clin. Oral Implant. Res. 2015, 26, 15–44. [Google Scholar] [CrossRef]
- D’Addazio, G.; Santilli, M.; Sinjari, B.; Xhajanka, E.; Rexhepi, I.; Mangifesta, R.; Caputi, S. Access to Dental Care-A Survey from Dentists, People with Disabilities and Caregivers. Int. J. Environ. Res. Public Health 2021, 18, 1556. [Google Scholar] [CrossRef]
- Aguirre-Zorzano, L.A.; Estefania-Fresco, R.; Telletxea, O.; Bravo, M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin. Oral Implant. Res. 2015, 26, 1338–1344. [Google Scholar] [CrossRef]
- Garcia-Bellosta, S.; Bravo, M.; Subira, C.; Echeverria, J.J. Retrospective study of the long-term survival of 980 implants placed in a periodontal practice. Int. J. Oral Maxillofac. Implant. 2010, 25, 613–619. [Google Scholar]
- Goh, M.S.; Hong, E.J.; Chang, M. Prevalence and risk indicators of peri-implantitis in Korean patients with a history of periodontal disease: A cross-sectional study. J. Periodontal Implant Sci. 2017, 47, 240–250. [Google Scholar] [CrossRef]
- Seki, K.; HaÃäkansson, J. Implant Treatment with 12-Year Follow-Up in a Patient with Severe Chronic Periodontitis: A Case Report and Literature Review. Case Rep. Dent. 2019, 2019, 3715159. [Google Scholar] [CrossRef]
- Spinato, S.; Bernardello, F.; Lombardi, T.; Soardi, C.M.; Messina, M.; Zaffe, D.; Stacchi, C. Influence of apico-coronal positioning of tissue-level implants on marginal bone stability during supracrestal tissue height establishment: A multi-center prospective study. Clin. Implant. Dent. Relat. Res. 2022, 24, 611–620. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shin, K.S.; Jung, J.H.; Cho, H.W.; Kwon, K.H.; Kim, Y.L. Clinical study on screw loosening in dental implant prostheses: A 6-year retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 133–142. [Google Scholar] [CrossRef]
- Jemt, T.; Laney, W.R.; Harris, D.; Henry, P.J.; Krogh, P.H., Jr.; Polizzi, G.; Zarb, G.A.; Herrmann, I. Osseointegrated implants for single tooth replacement: A 1-year report from a multicenter prospective study. Int. J. Oral Maxillofac. Implant. 1991, 6, 29–36. [Google Scholar]
- Kreissl, M.E.; Gerds, T.; Muche, R.; Heydecke, G.; Strub, J.R. Technical complications of implant-supported fixed partial dentures in partially edentulous cases after an average observation period of 5 years. Clin. Oral Implant. Res. 2007, 18, 720–726. [Google Scholar] [CrossRef]
- Cho, S.C.; Small, P.N.; Elian, N.; Tarnow, D. Screw loosening for standard and wide diameter implants in partially edentulous cases: 3- to 7-year longitudinal data. Implant Dent. 2004, 13, 245–250. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Bardini, L.; Sinjari, B.; Traini, T.; Marinelli, L.; Carraro, M.; Germani, R.; Di Profio, P.; Caputi, S.; Di Stefano, A.; et al. Surfactant hydrogels for the dispersion of carbon-nanotube-based catalysts. Chemistry 2013, 19, 16415–16423. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, K.; Braem, A.; Gerits, E.; De Brucker, K.; Vandamme, K.; Martens, J.A.; Michiels, J.; Vleugels, J.; Cammue, B.P.; Thevissen, K. Controlled Release of Chlorhexidine from a Mesoporous Silica-Containing Macroporous Titanium Dental Implant Prevents Microbial Biofilm Formation. Eur. Cells Mater. 2017, 33, 13–27. [Google Scholar] [CrossRef] [PubMed]





| Total patient number | 32 | ||
| Patients evaluated at 5-year follow-up | 31 | ||
| Patients in Group A | 16 | ||
| Patients in Group B | 15 | ||
| Implant failures | 1 (Group A) implant removed | ||
| Survival rate | 96.7% | ||
| Patients under strict hygienic control (PUC) | 58.1% | ||
| Global Full-Mouth Plaque Score (FMPS) | 21.38% ± 5.65 | ||
| Global Full-Mouth Bleeding Score (FMBS) | 20.96% ± 4.76 | ||
| Group A | Group B | Sig. | |
| FMPS (Group A–Group B) | 22.59% ± 5.93 | 19.83% ± 5.22 | p = 0.18 ns |
| FMBS (Group A–Group B) | 21.37% ± 5.46 | 20.39% ± 4.23 | p = 0.59 ns |
| FMPS (PUC/NO-PUC) | 17.81% ± 3.37 | 26.32% ± 4.42 | **** |
| FMBS (PUC/NO-PUC) | 18.17% ± 2.88 | 24.95% ± 4.3 | *** |
| ID PAT | SITE | GROUP | PUC/ NO-PUC | T0 | T1 | T2 | T3 | T4 | T5 | FMPS | FMBS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 16 | A | NO-PUC | 0.22 | −0.06 | −0.49 | −0.73 | −1.51 | −1.6 | 22.4 | 24.5 |
| 5 | 14 | A | PUC | 0 | −0.38 | −0.55 | −0.82 | −1.07 | −1.1 | 21.3 | 19.45 |
| 7 | 46 | A | PUC | 0.49 | 0.12 | −0.2 | −0.84 | −0.95 | −1.2 | 24.5 | 17.54 |
| 11 | 16 | A | NO-PUC | −0.62 | −1.53 | −1.75 | −1.76 | −1.84 | −2.2 | 25.6 | 18.34 |
| 14 | 47 | A | PUC | −0.39 | −0.92 | −0.89 | −0.65 | −0.8 | −0.87 | 19.3 | 18.5 |
| 17 | 36 | A | NO-PUC | −0.06 | −0.38 | −0.43 | −0.68 | −0.73 | −0.88 | 29.4 | 28.76 |
| 20 | 36 | A | PUC | 0.06 | 0 | −0.44 | −0.6 | −0.63 | −0.7 | 16.6 | 19.5 |
| 21 | 46 | A | PUC | 0.16 | −0.29 | −0.53 | −0.73 | −0.94 | −0.9 | 14.3 | 14.5 |
| 23 | 46 | A | NO-PUC | −0.13 | −0.2 | −0.74 | −1.01 | −1.05 | −1.68 | 31.4 | 29.04 |
| 24 | 35 | A | NO-PUC | −0.06 | −0.2 | −0.44 | −0.59 | −0.64 | −1.5 | 30.7 | 31.84 |
| 25 | 22 | A | PUC | 0.6 | −0.06 | −0.46 | −0.71 | −0.77 | −0.82 | 24.3 | 19.12 |
| 29 | 36 | A | PUC | −0.11 | −0.15 | −0.78 | −0.94 | −1.06 | −1.25 | 17.5 | 18.76 |
| 30 | 37 | A | PUC | 0.05 | −0.09 | −0.25 | −0.38 | −0.58 | −0.87 | 15.4 | 14.45 |
| 32 | 24 | A | NO-PUC | 0.35 | −0.05 | −0.52 | −0.79 | −0.9 | −1.1 | 29.87 | 27.12 |
| 33 | 36 | A | PUC | 0.21 | −0.05 | −0.4 | −0.58 | −0.74 | −0.74 | 16.3 | 19.09 |
| 34 | 46 | A | NO-PUC | −0.08 | −0.21 | −0.58 | −0.61 | −0.8 | REMOVED | 26.5 | 23.45 |
| 1 | 36 | B | NO-PUC | 0.57 | 0.62 | 0 | −0.47 | −0.55 | −1 | 25.8 | 26.09 |
| 4 | 36 | B | PUC | −0.17 | 0.03 | −0.5 | −0.71 | −0.81 | −1.32 | 14.6 | 21.45 |
| 8 | 46 | B | NO-PUC | 0.2 | 0.21 | −0.48 | −0.74 | −0.79 | −1.2 | 29.45 | 24.59 |
| 10 | 36 | B | PUC | −0.04 | 0 | −0.38 | −0.53 | −0.61 | −0.6 | 20.5 | 19.4 |
| 12 | 24 | B | PUC | 0 | 0.1 | −0.35 | −0.55 | −0.71 | −1.1 | 20.4 | 22.22 |
| 13 | 36 | B | NO-PUC | −0.02 | 0.15 | −0.37 | −0.67 | −0.89 | −1.25 | 19.3 | 18.41 |
| 15 | 36 | B | NO-PUC | 0.16 | 0.39 | −0.08 | −0.61 | −0.74 | −1.45 | 17.9 | 19.45 |
| 16 | 46 | B | PUC | 0.12 | 0.59 | −0.23 | −0.49 | −0.61 | −0.72 | 15.9 | 18.54 |
| 18 | 15 | B | PUC | 0 | −0.36 | −0.57 | −0.69 | −0.77 | −0.8 | 14.1 | 13.4 |
| 19 | 46 | B | PUC | 0.34 | 0.24 | 0 | −0.16 | −0.31 | −0.56 | 14.5 | 12.23 |
| 22 | 24 | B | PUC | 0.29 | 0.28 | −0.29 | −0.88 | −0.95 | −0.23 | 14.5 | 16.98 |
| 26 | 45 | B | PUC | −0.35 | −0.08 | −0.15 | −0.41 | −0.66 | −1.12 | 16.4 | 19.5 |
| 27 | 37 | B | NO-PUC | 0 | −0.05 | −0.21 | −0.54 | −0.59 | −0.62 | 27.8 | 24.98 |
| 28 | 25 | B | PUC | 0.06 | 0.18 | −0.3 | −0.37 | −0.57 | −0.72 | 20.12 | 22.45 |
| 31 | 26 | B | NO-PUC | −0.26 | −0.08 | −0.46 | −0.56 | −0.7 | −1.1 | 26.23 | 26.23 |
| MEAN | −0.8151613 mm | −1.04 mm | 21.38% | 20.96 % | |||||||
| ST. Dev | 0.28814199 | 0.39673669 | 5.65255588 | 4.76767566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Addazio, G.; Manciocchi, E.; Tafuri, G.; Schiavone, R.; Murmura, G.; Mavriqi, L.; Sinjari, B.; Caputi, S. Long-Term Efficacy of Chlorhexidine Gel in Single-Crown Implant Rehabilitation: A Five-Year Follow-Up Study. Dent. J. 2023, 11, 228. https://doi.org/10.3390/dj11100228
D’Addazio G, Manciocchi E, Tafuri G, Schiavone R, Murmura G, Mavriqi L, Sinjari B, Caputi S. Long-Term Efficacy of Chlorhexidine Gel in Single-Crown Implant Rehabilitation: A Five-Year Follow-Up Study. Dentistry Journal. 2023; 11(10):228. https://doi.org/10.3390/dj11100228
Chicago/Turabian StyleD’Addazio, Gianmaria, Eugenio Manciocchi, Giuseppe Tafuri, Ruggero Schiavone, Giovanna Murmura, Luan Mavriqi, Bruna Sinjari, and Sergio Caputi. 2023. "Long-Term Efficacy of Chlorhexidine Gel in Single-Crown Implant Rehabilitation: A Five-Year Follow-Up Study" Dentistry Journal 11, no. 10: 228. https://doi.org/10.3390/dj11100228
APA StyleD’Addazio, G., Manciocchi, E., Tafuri, G., Schiavone, R., Murmura, G., Mavriqi, L., Sinjari, B., & Caputi, S. (2023). Long-Term Efficacy of Chlorhexidine Gel in Single-Crown Implant Rehabilitation: A Five-Year Follow-Up Study. Dentistry Journal, 11(10), 228. https://doi.org/10.3390/dj11100228