Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
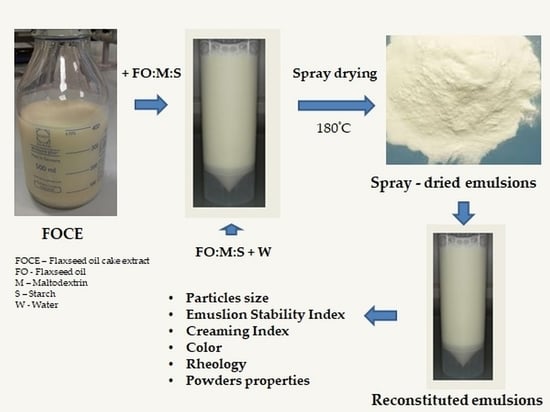
2.2. Emulsions Preparation
2.3. Spray Drying Protocol
2.4. Powders Characterization
2.4.1. Solubility and Total Solid Content
2.4.2. Bulk and Tapped Densities
2.4.3. Flowability and Cohesiveness of Powders
2.5. Emulsions Reconstitution and Characterization
2.5.1. Determination of Particle Size Changes in the Emulsion Samples and Optical Microscope Observations of Emulsions
2.5.2. Emulsions Stability
2.5.3. Emulsion Color Measurements
2.5.4. Emulsions Rheological Measurements
2.6. Statistical Analyses
3. Results and Discussion
3.1. Powders Characteristics
3.2. Emulsion Characteristics
3.2.1. Emulsion Particles Size Changes
3.2.2. Emulsion Stability
3.2.3. Color of Emulsions
3.2.4. Emulsion Rheological Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koç, M.; Güngör, Ö.; Zungur, A.; Yalçın, B.; Selek, İ.; Ertekin, F.K.; Ötles, S. Microencapsulation of Extra Virgin Olive Oil by Spray Drying: Effect of Wall Materials Composition, Process Conditions, and Emulsification Method. Food Bioprocess Technol. 2015, 8, 301–318. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Influence of pH and carrageenan type on properties of β-lactoglobulin stabilized oil-in-water emulsions. Food Hydrocoll. 2005, 19, 83–91. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Spray drying of mono- and double-layer emulsions of PUFA-rich vegetable oil homogenized by ultrasound. Dry. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Wang, B.; Tian, H.; Xiang, D. Stabilizing the Oil-in-Water Emulsions Using the Mixtures of Dendrobium Officinale Polysaccharides and Gum Arabic or Propylene Glycol Alginate. Molecules 2020, 25, 759. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, X.M.; Guo, S.D. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The effect of native and denaturated flaxseed meal extract on physiochemical properties of low fat mayonnaises. J. Food Meas. Charact. 2020, 14, 1135–1145. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Oomah, B.D.; Mazza, G. Flaxseed proteins-a review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Dong, X.; Du, S.; Deng, Q.; Tang, H.; Yang, C.; Wei, F.; Chen, H.; Quek, S.Y.; Zhou, A.; Liu, L. Study on the antioxidant activity and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int. J. Biol. Macromol. 2020, 153, 1157–1164. [Google Scholar] [CrossRef]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000.
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2015, 170, 33–40. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Carr, L.R. Evaluating Flow Properties of Solids. Chem. Eng. 1965, 18, 163–168. [Google Scholar]
- Hausner, H.H. Friction conditions in a mass of metal powder. Int. J. Powder Metall. 1967, 3, 7–13. [Google Scholar]
- Reddy, R.S.; Ramachandra, C.T.; Hiregoudar, S.; Nidoni, U.; Ram, J.; Kammar, M. Influence of processing conditions on functional and reconstitution properties of milk powder made from Osmanabadi goat milk by spray drying. Small Rumin. Res. 2014, 119, 130–137. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.J.; Adhikari, B. The effect of addition of flaxseed gum on the emulsion properties of soybean protein isolate (SPI). J. Food Eng. 2011, 104, 56–62. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClements, D.J. Stability and rheology of corn oil-in-water emulsions containing maltodextrin. Food Res. Int. 2004, 37, 851–859. [Google Scholar] [CrossRef]
- Cameron, D.R.; Weber, M.E.; Idziak, E.S.; Neufeld, R.J.; Cooper, D.G. Determination of Inter facial Areas in Emulsions Using Turbidimetric and Droplet Size Data: Correction of the Formula for Emulsifying Activity Index. J. Agric. Food Chem. 1991, 655–659. [Google Scholar] [CrossRef]
- Kumar, R.G.L.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.R.F.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- McClements, D.J. Colloidal basis of emulsion color. Curr. Opin. Colloid Interface Sci. 2002, 7, 451–455. [Google Scholar] [CrossRef]
- Aizawa, H. Novel Pragmatic Turbidimetric Data Analysis Method for Evaluating the Stability of Emulsions. Int. J. Food Prop. 2014, 17, 1264–1274. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M. Depletion flocculation of emulsions containing unadsorbed sodium caseinate. Food Hydrocoll. 1997, 11, 13–18. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Dokic-Baucal, L.; Dokic, P.; Jakovljevic, J. Influence of different maltodextrins on properties of O/W emulsions. Food Hydrocoll. 2004, 18, 233–239. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Creaming stability of flocculated monodisperse oil-in-water emulsions. J. Colloid Interface Sci. 2000, 225, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kupongsak, S.; Sathitvorapojjana, S. Properties and Storage Stability of O/W Emulsion Replaced with Medium-Chain Fatty Acid Oil. Polish J. Food Nutr. Sci. 2017, 67, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Ge, Z.Z.; Xu, Z.; Zou, B.; Zhang, Y.; Li, C.M. Comparison of the Efficiency of Five Different Drying Carriers on the Spray Drying of Persimmon Pulp Powders. Dry. Technol. 2014, 32, 1157–1166. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Okuro, P.K.; Santos, M.; de Paglarini, C.S.; da Cunha, R.L.; Ruiz-Capillas, C.; Herrero, A.M.; Pollonio, M.A.R. Understanding the role of chia (Salvia hispanica L.) mucilage on olive oil-based emulsion gels as a new fat substitute in emulsified meat products. Eur. Food Res. Technol. 2020, 246, 909–922. [Google Scholar] [CrossRef]
- Verruck, S.; de Liz, G.R.; Dias, C.O.; de Mello Castanho Amboni, R.D.; Prudencio, E.S. Effect of full-fat goat’s milk and prebiotics use on Bifidobacterium BB-12 survival and on the physical properties of spray-dried powders under storage conditions. Food Res. Int. 2019, 119, 643–652. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Truong, T.; Adhikari, B. Microencapsulation of flaxseed oil using polyphenol-adducted flaxseed protein isolate-flaxseed gum complex coacervates. Food Hydrocoll. 2020, 107, 105944. [Google Scholar] [CrossRef]
- Campelo, P.H.; Junqueira, L.A.; de Resende, J.V.; Zacarias, R.D.; de Fernandes, R.V.B.; Botrel, D.A.; Borges, S.V. Stability of lime essential oil emulsion prepared using biopolymers and ultrasound treatment. Int. J. Food Prop. 2017, 20, S564–S579. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Kurek, M.A.; Pratap-Singh, A. Plant-Based (Hemp, Pea and Rice) Protein–Maltodextrin Combinations as Wall Material for Spray-Drying Microencapsulation of Hempseed (Cannabis sativa) Oil. Foods 2020, 9, 1707. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of fish oil in soybean protein particles by emulsification and spray drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]





| Sample Name | Maltodextrin: Starch Ratio | Liquid Phase | Oil Content * |
|---|---|---|---|
| FOCE-MS-10% | 1:1 | FOCE | 10% |
| FOCE-MS-20% | 1:1 | FOCE | 20% |
| MS-10% | 1:1 | Distilled Water | 10% |
| MS-20% | 1:1 | Distilled Water | 20% |
| Carr’s Index | Hausner Ratio | |
|---|---|---|
| Excellent | 0–10% | 1.00–1.11 |
| Good | 10–15% | 1.12–1.18 |
| Fair | 16–20% | 1.19–1.25 |
| Possible | 21–25% | 1.26–1.34 |
| Poor | 26–31% | 1.35–1.45 |
| Very poor | 32–37% | 1.46–1.59 |
| Very very poor | >38% | >1.60 |
| Powder Sample | HR | C (%) | ||
|---|---|---|---|---|
| FOCE-MS-10% | 0.313 ± 0.001 b | 0.405 ± 0.004 c | 1.29 ± 0.01 a | 25.54 ± 0.49 a |
| FOCE-MS-20% | 0.370 ± 0.001 a | 0.432 ± 0.001 a | 1.17 ± 0.00 d | 14.29 ± 0.00 d |
| MS-10% | 0.351 ± 0.018 a | 0.424 ± 0.001 b | 1.21 ± 0.06 c | 17.14 ± 0.41 c |
| MS-20% | 0.306 ± 0.015 b | 0.347 ± 0.017 d | 1.23 ± 0.12 b | 18.01 ± 0.78 b |
| Powder Sample | Solubility (%) | Yield (%) | TSC (%) |
|---|---|---|---|
| FOCE-MS-10% | 94.71 ± 2.47 a | 44.26 ± 0.10 c | 98.11 ± 0.57 a |
| FOCE-MS-20% | 98.71 ± 1.87 ab | 46.58 ± 0.82 d | 98.68 ± 1.00 a |
| MS-10% | 98.61 ± 1.05 ab | 57.98 ± 0.53 a | 95.15 ± 0.15 b |
| MS-20% | 96.67 ± 2.00 b | 54.98 ± 0.42 b | 96.76 ± 1.00 b |
| Sample | FOCE-MS-10% | FOCE-MS-20% | MS-10% | MS-20% |
|---|---|---|---|---|
| D4.3 (µm) | ||||
| A | 3.225 ± 0.05 Aa | 2.700 ± 0.01 Ba | 3.206 ± 0.12 Cb | 3.045 ± 0.00 Db |
| B | 3.065 ± 0.01 Ab | 2.693 ± 0.00 Bb | 3.342 ± 0.00 Ca | 3.069 ± 0.01 Da |
| D3.2 (µm) | ||||
| A | 2.327 ± 0.02 Aa | 2.445 ± 0.02 Ba | 2.206 ± 0.05 Cb | 2.256 ± 0.01 Db |
| B | 2.271 ± 0.02 Ab | 2.166 ± 0.01 Bb | 2.280 ± 0.00 Ca | 2.305 ± 0.00 Da |
| Sample | FOCE-MS-10% | FOCE-MS-20% | MS-10% | MS-20% |
|---|---|---|---|---|
| L* | ||||
| A | 86.99 ± 0.00 Aa | 87.07 ± 0.00 Aa | 97.06 ± 0.00 Ca | 96.69 ± 0.00 Da |
| B | 84.42 ± 0.01 Ab | 86.92 ± 0.01 Bb | 83.28 ± 0.00 Cb | 87.70 ± 0.00 Db |
| a* | ||||
| A | −1.83 ± 0.01 Aa | −1.06 ± 0.01 Ba | −0.17 ± 0.01 Ca | −0.07 ± 0.01 Da |
| B | −2.33 ± 0.01 Ab | −1.64 ± 0.01 Bb | −1.81 ± 0.01 Cb | −1.18 ± 0.01 Db |
| b* | ||||
| A | 16.92 ± 0.01 Ab | 15.88 ± 0.02 Bb | 4.32 ± 0.02 Cb | 5.48 ± 0.02 Db |
| B | 19.91 ± 0.02 Aa | 17.37 ± 0.01 Ba | 9.08 ± 0.02 Ca | 15.54 ± 0.02 Da |
| YI | ||||
| A | 28.63 ± 0.01 Ab | 26.06 ± 0.12 Bb | 6.36 ± 0.01 Cb | 8.10 ± 0.01 Db |
| B | 32.70 ± 0.04 Aa | 28.55 ± 0.01 Ba | 15.58 ± 0.01 Ca | 25.31 ± 0.01 Da |
| WI | ||||
| A | 76.14 ± 0.02 Ab | 79.49 ± 0.02 Ba | 94.77 ± 0.00 Ca | 93.59 ± 0.00 Da |
| B | 76.88 ± 0.00 Aa | 78.19 ± 0.00 Bb | 80.88 ± 0.00 Cb | 80.15 ± 0.00 Db |
| ΔE | ||||
| A | Used as standard | Used as standard | Used as standard | Used as standard |
| B | 3.98 ± 0.01 A | 1.61 ± 0.07 B | 14.68 ± 0.01 C | 13.53 ± 0.01 D |
| Sample | FOCE-MS-10% | FOCE-MS-20% | MS-10% | MS-20% |
|---|---|---|---|---|
| τy (Pa) | ||||
| A | 0.0534 ± 0.000 Aa | 0.0520 ± 0.001 Ba | 0.0090 ± 0.003 Ca | 0.0026 ± 0.004 Da |
| B | 0.0225 ± 0.001 Ab | 0.0255 ± 0.000 Bb | 0.0030 ± 0.002 Cb | 0.0032 ± 0.002 Ca |
| Viscosity (Pa·s) | ||||
| A | 0.2710 ± 0.001 Aa | 0.2350 ± 0.001 Ba | 0.0450 ± 0.000 Ca | 0.0130 ± 0.001 Da |
| B | 0.0197 ± 0.002 Ab | 0.0248 ± 0.000 Bb | 0.0057 ± 0.001 Cb | 0.0145 ± 0.000 Db |
| k (Pa·sn) | ||||
| A | 0.1638 ± 0.005 Aa | 0.3310 ± 0.010 Ba | 0.0150 ± 0.002 Ca | 0.0141 ± 0.001 Da |
| B | 0.0204 ± 0.001 Ab | 0.0263 ± 0.005 Bb | 0.0057 ± 0.001 Cb | 0.0138 ± 0.003 Db |
| n (–) | ||||
| A | 0.48 ± 0.02 Ab | 0.95 ± 0.01 Bb | 0.78 ± 0.00 Cb | 0.85 ± 0.02 Db |
| B | 1.00 ± 0.00 Aa | 0.98 ± 0.02 Ba | 1.00 ± 0.00 Aa | 1.00 ± 0.00 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods 2021, 10, 256. https://doi.org/10.3390/foods10020256
Drozłowska E, Bartkowiak A, Trocer P, Kostek M, Tarnowiecka-Kuca A, Łopusiewicz Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods. 2021; 10(2):256. https://doi.org/10.3390/foods10020256
Chicago/Turabian StyleDrozłowska, Emilia, Artur Bartkowiak, Paulina Trocer, Mateusz Kostek, Alicja Tarnowiecka-Kuca, and Łukasz Łopusiewicz. 2021. "Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent" Foods 10, no. 2: 256. https://doi.org/10.3390/foods10020256
APA StyleDrozłowska, E., Bartkowiak, A., Trocer, P., Kostek, M., Tarnowiecka-Kuca, A., & Łopusiewicz, Ł. (2021). Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods, 10(2), 256. https://doi.org/10.3390/foods10020256