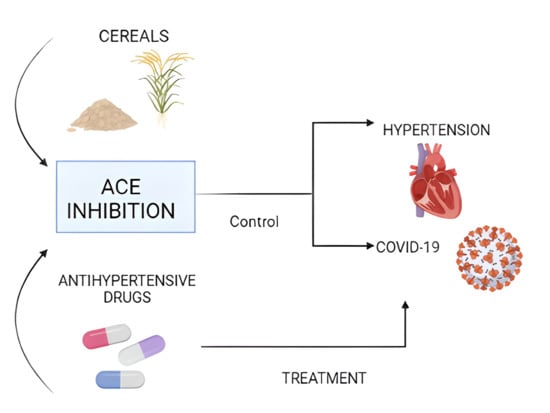
Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19
Abstract
:1. Introduction
2. Physiopathology of Hypertension
3. Hypertension: Main Comorbidity in Patients with COVID-19
4. Anti-Hypertensive Drugs and Their Use in the Treatment of COVID-19
5. Cereals as a Source of Compounds with Anti-Hypertensive Activity
5.1. Rice
5.2. Barley
5.3. Corn
5.4. Wheat
5.5. Oats
5.6. Millet
5.7. Rye
5.8. Sorghum
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Björck, I.; Östman, E.; Kristensen, M.; Mateo Anson, N.; Price, R.K.; Haenen, G.R.M.M.; Havenaar, R.; Bach Knudsen, K.E.; Frid, A.; Mykkänen, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Technol. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Mhd Omar, N.A.; Brunius, C.; Hallmans, G.; Johansson, J.-E.; Andersson, S.-O.; Larsson, A.; Åman, P.; Landberg, R. Consumption of whole grain/bran rye instead of refined wheat decrease concentrations of TNF-R2, e-selectin, and endostatin in an exploratory study in men with prostate cancer. Clin. Nutr. 2020, 39, 159–165. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Ereifej, K.; Abu-Zaiton, A.; Alrababah, M.; Almajwal, A.; Rababah, T.; Yang, W. Anti-oxidant, anti-diabetic, and anti-hypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions. Int. J. Food Prop. 2012, 15, 781–795. [Google Scholar] [CrossRef]
- Gupta, R.; Meghwal, M.; Prabhakar, P.K. Bioactive compounds of pigmented wheat (Triticum aestivum): Potential benefits in human health. Trends Food Sci. Technol. 2021, 110, 240–252. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Woo, S.; Kim, M.-J.; Kwon, S.-W.; Lee, J.; Sung, S.H.; Koh, H.-J. Identification and quantification of flavonoids in yellow grain mutant of rice (Oryza sativa L.). Food Chem. 2018, 241, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Cavazos, A.; Gonzalez de Mejia, E. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komolafe, K.; Akinmoladun, A.C.; Komolafe, T.R.; Olaleye, M.T.; Boligon, A.A.; Akindahunsi, A.A.; Rocha, J.B.T. Angiotensin-1-converting enzyme inhibition, antioxidant activity, and modulation of cerebral Na+/K+ ATPase by free phenolics of African locust bean (Parkia biglobosa). Health Sci. Rep. 2017, 1, e17. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281.e6–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.J.; Ye, M.; Wysocki, J.; William, J.; Lloveras, J.; Batlle, D. Localization of ACE2 in the renal vasculature: Amplification by angiotensin II type 1 receptor blockade using telmisartan. Am. J. Physiol. Ren. Physiol. 2009, 296, F398–F405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ye, Y.; Gong, H.; Wu, J.; Yuan, J.; Wang, S.; Yin, P.; Ding, Z.; Kang, L.; Jiang, Q.; et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1–7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016, 97, 180–190. [Google Scholar] [CrossRef]
- Baptiste, D.-L.; Hamilton, J.B.; Foronda, C.; Sloand, E.; Fahlberg, B.; Pfaff, T.; Delva, S.; Davidson, P.M. Hypertension among adults living in Haiti: An integrative review. J. Clin. Nurs. 2018, 27, 2536–2545. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539859/ (accessed on 27 January 2021).
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Larsen, M.K.; Matchkov, V.V. Hypertension and physical exercise: The role of oxidative stress. Medicina 2016, 52, 19–27. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, K.; Majumder, K. Chapter Four—Food-derived bioactive peptides and their role in ameliorating hypertension and associated cardiovascular diseases. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 89, pp. 165–207. ISBN 1043-4526. [Google Scholar]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Auger, C.; Kurita, I.; Anselm, E.; Rivoarilala, L.O.; Lee, H.J.; Lee, K.W.; Schini-Kerth, V.B. Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide 2013, 35, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Taleb, H.; Morris, K.; Withycombe, C.E.; Maddocks, S.E.; Kanekanian, A.D. Date syrup–derived polyphenols attenuate angiogenic responses and exhibits anti-inflammatory activity mediated by vascular endothelial growth factor and cyclooxygenase-2 expression in endothelial cells. Nutr. Res. 2016, 36, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Cheng, Y. Increasing Host Cellular Receptor—Angiotensin-Converting Enzyme 2 (ACE2) Expression by Coronavirus may Facilitate 2019-nCoV Infection. bioRxiv 2020, 92, 2696–2701. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. bioRxiv 2020, 202, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Hwang, S.W. Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception. Biomol. Ther. 2018, 26, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Maddox, T.M.; Messerli, F.H. Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiol. 2020, 5, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Devaux, C.A.; Rolain, J.-M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef]
- Furuhashi, M.; Moniwa, N.; Mita, T.; Fuseya, T.; Ishimura, S.; Ohno, K.; Shibata, S.; Tanaka, M.; Watanabe, Y.; Akasaka, H.; et al. Urinary Angiotensin-Converting Enzyme 2 in Hypertensive Patients May Be Increased by Olmesartan, an Angiotensin II Receptor Blocker. Am. J. Hypertens. 2015, 28, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Mumford, S.L.; Rovner, A.J.; Zhang, C.; Chen, L.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F.; BioCycle Study Group. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J. Nutr. 2010, 140, 1669–1676. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Goletzke, J.; Buyken, A.E.; Joslowski, G.; Bolzenius, K.; Remer, T.; Carstensen, M.; Egert, S.; Nöthlings, U.; Rathmann, W.; Roden, M.; et al. Increased Intake of Carbohydrates from Sources with a Higher Glycemic Index and Lower Consumption of Whole Grains during Puberty Are Prospectively Associated with Higher IL-6 Concentrations in Younger Adulthood among Healthy Individuals. J. Nutr. 2014, 144, 1586–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herder, C.; Peltonen, M.; Koenig, W.; Sütfels, K.; Lindström, J.; Martin, S.; Ilanne-Parikka, P.; Eriksson, J.G.; Aunola, S.; Keinänen-Kiukaanniemi, S.; et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 2009, 52, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braude, P.; Carter, B.; Short, R.; Vilches-Moraga, A.; Verduri, A.; Pearce, L.; Price, A.; Quinn, T.J.; Stechman, M.; Collins, J.; et al. The influence of ACE inhibitors and ARBs on hospital length of stay and survival in people with COVID-19. IJC Heart Vasc. 2020, 31, 100660. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Misra, A. Comorbidities in COVID-19: Outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-del Río, J.; Piqueras-Flores, J.; Nieto-Sandoval Martín de la Sierra, P.; Negreira-Caamaño, M.; Águila-Gordo, D.; Mateo-Gómez, C.; Salas-Bravo, D.; Rodríguez-Martínez, M. Análisis de la relación entre los inhibidores del sistema renina-angiotensina y la evolución de pacientes hospitalizados por infección respiratoria COVID-19. Med. Clínica 2020, 155, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infe. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar] [CrossRef]
- Reddy, R.; Asante, I.; Liu, S.; Parikh, P.; Liebler, J.; Borok, Z.; Rodgers, K.; Baydur, A.; Louie, S.G. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS ONE 2019, 14, e0213096. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271.e8–280.e8. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Du, Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zheng, W.; Cheng, L.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus Fruits Are Rich in Flavonoids for Immunoregulation and Potential Targeting ACE2. Natural Products and Bioprospecting 2022, 12, 4. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Shen, X.; Cao, Y.-K.; Zhang, J.-J.; Wang, Y.; Cheng, Y.-X. Discovery of Anti-2019-nCoV Agents from Chinese Patent Drugs via Docking Screening. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Chourasia, R.; Padhi, S.; Chiring Phukon, L.; Abedin, M.M.; Singh, S.P.; Rai, A.K. A Potential Peptide from Soy Cheese Produced Using Lactobacillus delbrueckii WS4 for Effective Inhibition of SARS-CoV-2 Main Protease and S1 Glycoprotein. Front. Mol. Biosci. 2020, 7, 601753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, X.; Yu, Z.; Wu, S.; Ding, L.; Liu, J. Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-CoV-2. LWT 2022, 154, 112684. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Borneo, R.; León, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110–119. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bächler, J.P. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef]
- Cian, R.E.; Caballero, M.S.; Sabbag, N.; González, R.J.; Drago, S.R. Bio-accessibility of bioactive compounds (ACE inhibitors and antioxidants) from extruded maize products added with a red seaweed Porphyra columbina. LWT Food Sci. Technol. 2014, 55, 51–58. [Google Scholar] [CrossRef]
- Jan-on, G.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Sattayasai, J.; Senaphan, K.; Kukongviriyapan, U. Virgin rice bran oil alleviates hypertension through the upregulation of eNOS and reduction of oxidative stress and in flammation in L-NAME À induced hypertensive rats. Nutrition 2020, 69, 110575. [Google Scholar] [CrossRef] [PubMed]
- Massaretto, I.L.; Madureira Alves, M.F.; Mussi de Mira, N.V.; Carmona, A.K.; Lanfer Marquez, U.M. Phenolic compounds in raw and cooked rice (Oryza sativa L.) and their inhibitory effect on the activity of angiotensin I-converting enzyme. J. Cereal Sci. 2011, 54, 236–240. [Google Scholar] [CrossRef]
- Pannangpetch, P.; Tangsucharit, P.; Thanaruksa, R.; Proongkhong, T.; Srisuwan, S.; Aekthammarat, D. Antihypertensive effect of Mali-Nil surin rice bran hydrolysate and its mechanisms related to the EDHF-mediated vasorelaxation and L-type Ca2+ channel-mediated vasoconstriction in L-NAME hypertensive rats. Biomed. Pharmacother. 2022, 150, 113003. [Google Scholar] [CrossRef]
- Ra, J.E.; Woo, S.Y.; Jin, H.; Lee, M.J.; Kim, H.Y.; Ham, H.; Chung, I.M. Evaluation of antihypertensive polyphenols of barley (Hordeum vulgare L.) seedlings via their effects on angiotensin—Converting enzyme (ACE) inhibition. Appl. Biol. Chem. 2020, 63, 38. [Google Scholar] [CrossRef]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Food Sci. Technol. 2013, 48, 984–991. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.; Mheiri, A.A.; Abushelaibi, A. Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented lupin, quinoa and wheat by Bifidobacterium species: In-vitro investigations. LWT-Food Sci. Technol. 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Reque, P.M.; Orlandini Werner, J.A.; Barreto Pinilla, C.M.; Folmer Corrêa, A.P.; Rodrigues, E.; Brandelli, A. Biological activities of wheat middlings bioprocessed with Bacillus spp. LWT 2017, 77, 525–531. [Google Scholar] [CrossRef]
- Irondi, E.A.; Adegoke, B.M.; Effion, E.S.; Oyewo, S.O.; Alamu, E.O.; Boligon, A.A. Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Hum. Wellness 2019, 8, 142–148. [Google Scholar] [CrossRef]
- Arouna, N.; Gabriele, M.; Pucci, L. The Impact of Germination on Sorghum Nutraceutical Properties. Foods 2020, 9, 1218. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Health Benefits of Traditional Corn, Beans, and Pumpkin: In Vitro Studies for Hyperglycemia and Hypertension Management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of Chain Length on Absorption of Biologically Active Peptides from the Gastrointestinal Tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef]
- Aluko, R.E. Structure and function of plant protein-derived antihypertensive peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]
- Zhang, P.; Chang, C.; Liu, H.; Li, B.; Yan, Q.; Jiang, Z. Identification of novel angiotensin I-converting enzyme (ACE) inhibitory peptides from wheat gluten hydrolysate by the protease of Pseudomonas aeruginosa. J. Funct. Foods 2020, 65, 103751. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Wynne, K.; Connor, P.O.; Gallagher, E.; Brunton, N.P.; Rai, D.K.; Hayes, M. In silico and in vitro analyses of the angiotensin-I converting enzyme inhibitory activity of hydrolysates generated from crude barley (Hordeum vulgare) protein concentrates. Food Chem. 2016, 203, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Su, K.; Zhang, X. Potential of Plant Proteins Digested In Silico by Gastrointestinal Enzymes as Nutritional Supplement for COVID-19 Patients. Plant Foods Hum. Nutr. 2020, 75, 583–591. [Google Scholar] [CrossRef]
- Ejike, C.E.C.C.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D. Plant food-derived Angiotensin I converting enzyme inhibitory peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef] [PubMed]
- Michelke, L.; Deussen, A.; Dieterich, P.; Martin, M. Effects of bioactive peptides encrypted in whey-, soy- and rice protein on local and systemic angiotensin-converting enzyme activity. J. Funct. Foods 2017, 28, 299–305. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, K.; Wu, B.; Wu, P.; Duan, Y.; Ma, H. Production of ACE inhibitory peptides from corn germ meal by an enzymatic membrane reactor with a novel gradient diafiltration feeding working-mode and in vivo evaluation of antihypertensive effect. J. Funct. Foods 2020, 64, 103584. [Google Scholar] [CrossRef]
- Wu, D.; Ren, J.; Song, C. Optimization of Enzymatic Hydrolysis of Corn Germ Meal to Prepare ACE Inhibitory Peptides. Sci. Technol. Cereals Oils Foods 2014, 22, 51–53. [Google Scholar]
- Huang, W.H.; Sun, J.; He, H.; Dong, H.W.; Li, J.T. Antihypertensive effect of corn peptides, produced by a continuous production in enzymatic membrane reactor, in spontaneously hypertensive rats. Food Chem. 2011, 128, 968–973. [Google Scholar] [CrossRef]
- Lin, F.; Chen, L.; Liang, R.; Zhang, Z.; Wang, J.; Cai, M.; Li, Y. Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro. Food Chem. 2011, 124, 801–807. [Google Scholar] [CrossRef]
- Asoodeh, A.; Haghighi, L.; Chamani, J.; Ansari-Ogholbeyk, M.A.; Mojallal-Tabatabaei, Z.; Lagzian, M. Potential angiotensin I converting enzyme inhibitory peptides from gluten hydrolysate: Biochemical characterization and molecular docking study. J. Cereal Sci. 2014, 60, 92–98. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Zhao, W.; Pan, Z. ACE-inhibitory peptides production from defatted wheat germ protein by continuous coupling of enzymatic hydrolysis and membrane separation: Modeling and experimental studies. Chem. Eng. J. 2013, 226, 139–145. [Google Scholar] [CrossRef]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Gammoh, S.; Alu’datt, M.H.; Alhamad, M.N.; Rababah, T.; Al-Mahasneh, M.; Qasaimeh, A.; Johargy, A.; Kubow, S.; Hussein, N.M. The effects of protein-phenolic interactions in wheat protein fractions on allergenicity, antioxidant activity and the inhibitory activity of angiotensin I-converting enzyme (ACE). Food Biosci. 2018, 24, 50–55. [Google Scholar] [CrossRef]
- Wang, B.; Atungulu, G.G.; Khir, R. Ultrasonic Treatment Effect on Enzymolysis Kinetics and Activities of ACE-Inhibitory Peptides from Oat-Isolated Protein. Food Biophysics. 2015, 10, 244–252. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’ Shea, N.; Gallagher, E.; Lafarga, T. Predicted Release and Analysis of Novel ACE-I, Renin, and DPP-IV Inhibitory Peptides from Common Oat (Avena sativa) Protein Hydrolysates Using in Silico Analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, I.W.Y.; Nakayama, S.; Hsu, M.N.K.; Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Angiotensin-I Converting Enzyme Inhibitory Activity of Hydrolysates from Oat (Avena sativa) Proteins by In Silico and In Vitro Analyses. J. Agric. Food Chem. 2009, 57, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Du, J.; Jia, J.; Kuang, C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016, 199, 140–149. [Google Scholar] [CrossRef]
- Kamath, V.; Niketh, S.; Chandrashekar, A.; Rajini, P.S. Chymotryptic hydrolysates of α-kafirin, the storage protein of sorghum (Sorghum bicolor) exhibited angiotensin converting enzyme inhibitory activity. Food Chem. 2007, 100, 306–311. [Google Scholar] [CrossRef]
- Zhao, C.J.; Hu, Y.; Schieber, A.; Gänzle, M. Fate of ACE-inhibitory peptides during the bread-making process: Quanti fi cation of peptides in sourdough, bread crumb, steamed bread and soda crackers. J. Cereal Sci. 2013, 57, 514–519. [Google Scholar] [CrossRef]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2017, 56, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Chen, J.; Ren, X.; Wang, C.; Diao, X.; Hu, X.; Zhang, Y.; Shen, Q. A whole foxtail millet diet reduces blood pressure in subjects with mild hypertension. J. Cereal Sci. 2018, 84, 13–19. [Google Scholar] [CrossRef]
- Gong, E.S.; Liu, C.; Li, B.; Zhou, W.; Chen, H.; Li, T.; Wu, J.; Zeng, Z.; Wang, Y.; Si, X.; et al. Phytochemical profiles of rice and their cellular antioxidant activity against ABAP induced oxidative stress in human hepatocellular carcinoma HepG2 cells. Food Chem. 2020, 318, 126484. [Google Scholar] [CrossRef]
- Yu, X.; Chu, M.; Chu, C.; Du, Y.; Shi, J.; Liu, X.; Liu, Y.; Zhang, H.; Zhang, Z.; Yan, N. Wild rice (Zizania spp.): A review of its nutritional constituents, phytochemicals, antioxidant activities, and health-promoting effects. Food Chem. 2020, 331, 127293. [Google Scholar] [CrossRef] [PubMed]
- Okarter, N.; Liu, R.H. Health Benefits of Whole Grain Phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, Y.; Qian, B.; Liu, Z.; Zheng, Y.; Song, X.; Lai, S.; Zhao, Y. Antihypertensive effect of few-flower wild rice (Zizania latifolia Turcz.) in spontaneously hypertensive rats. Food Sci. Biotechnol. 2014, 23, 439–444. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liang, Y.; Bai, J.; Wu, W.; Lin, Q.; Wu, J. Spent hen-derived ACE inhibitory peptide IWHHT shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2019, 53, 85–92. [Google Scholar] [CrossRef]
- Kopaliani, I.; Martin, M.; Zatschler, B.; Müller, B.; Deussen, A. Whey peptide Isoleucine-Tryptophan inhibits expression and activity of matrix metalloproteinase-2 in rat aorta. Peptides 2016, 82, 52–59. [Google Scholar] [CrossRef]
- Lunow, D.; Kaiser, S.; Rückriemen, J.; Pohl, C.; Henle, T. Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme. Food Chem. 2015, 166, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, N.B.; Yılmaz, N. Gamma-oryzanol content, phenolic acid profiles and antioxidant activity of rice milling fractions. Eur. Food Res. Technol. 2011, 233, 577. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2016, 25, 148–161. [Google Scholar] [CrossRef] [Green Version]
- FDA. New Hope Network. Available online: https://www.newhope.com/supply-news-amp-analysis/fda-finalizes-health-claim-associating-consumption-barley-products-reductio (accessed on 4 October 2021).
- Topping, D.; Morell, M. Chapter 9—Barley foods and public health. In American Associate of Cereal Chemists International, 2nd ed.; Shewry, P.R., Ullrich, S.E.B.T.-B., Eds.; AACC International Press: Washington, DC, USA, 2014; pp. 223–231. ISBN 978-1-891127-79-3. [Google Scholar]
- Huang, W.; Davidge, S.; Wu, J. Bioactive Natural Constituents from Food Sources—Potential Use in Hypertension Prevention and Treatment. Crit. Rev. Food Sci. Nutr. 2013, 53, 615–630. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT-Food Sci. Technol. 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Kinitz, C.; Knutsen, S.H. Flavanol and Bound Phenolic Acid Contents in Different Barley Varieties. J. Agric. Food Chem. 2006, 54, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Hyun, J.-N.; Kim, J.-A.; Park, J.-C.; Kim, M.-Y.; Kim, J.-G.; Lee, S.-J.; Chun, S.-C.; Chung, I.-M. Relationship between Phenolic Compounds, Anthocyanins Content and Antioxidant Activity in Colored Barley Germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.A.M.; Lampi, A.-M.; Nyström, L.; Piironen, V.; Li, L.; Ward, J.L.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Boros, D.; et al. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9767–9776. [Google Scholar] [CrossRef]
- Bellido, G.G.; Beta, T. Anthocyanin composition and oxygen radical scavenging capacity (ORAC) of milled and pearled purple, black, and common barley. J. Agric. Food Chem. 2009, 57, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. J. Am. Diet. Assoc. 2006, 106, 1445–1449. [Google Scholar] [CrossRef]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.M.; Scott, H.A.; Wood, L.G. Soluble fibre as a treatment for inflammation in asthma. J. Nutr. Intermed. Metab. 2019, 18, 100108. [Google Scholar] [CrossRef]
- Collier, M.E.W.; Zhang, S.; Scrutton, N.S.; Giorgini, F. Inflammation control and improvement of cognitive function in COVID-19 infections: Is there a role for kynurenine 3-monooxygenase inhibition? Drug Discov. Today 2021, 26, 1473–1481. [Google Scholar] [CrossRef]
- Conte, L.; Toraldo, D.M. Targeting the gut–lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Adv. Respir. Dis. 2020, 14, 1753466620937170. [Google Scholar] [CrossRef]
- Abirami, S.; Priyalakshmi, M.; Soundariya, A.; Samrot, A.V.; Saigeetha, S.; Emilin, R.R.; Dhiva, S.; Inbathamizh, L. Antimicrobial activity, antiproliferative activity, amylase inhibitory activity and phytochemical analysis of ethanol extract of corn (Zea mays L.) silk. Curr. Res. Green Sustain. Chem. 2021, 4, 100089. [Google Scholar] [CrossRef]
- Andjelkovic, V.; Vukadinović, J.; Srebric, M.; Mladenović-Drinić, S. Pigmented maize—A potential source of β-carotene and α-tocopherol. J. Eng. Process. Manag. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Sánchez, G.S.; Hernández, D.R.; Lobato, N.R. Characterization of anthocyanin extracts from maize kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhang, C.; Guigas, C.; Ma, Y.; Corrales, M.; Tauscher, B.; Hu, X. Composition, antimicrobial activity, and antiproliferative capacity of anthocyanin extracts of purple corn (Zea mays L.) from China. Eur. Food Res. Technol. 2009, 228, 759–765. [Google Scholar] [CrossRef]
- Scott, C.E.; Eldridge, A.L. Comparison of carotenoid content in fresh, frozen and canned corn. J. Food Compos. Anal. 2005, 18, 551–559. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, J. Profiles of Carotenoids, Anthocyanins, Phenolics, and Antioxidant Activity of Selected Color Waxy Corn Grains during Maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Romani, F.I. Annalisa Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Psaltopoulou, T.; Kapetanstrataki, M.G.; Plytzanopoulou, P.; Vassilakou, T.; Malamos, N.; Linos, A.; Panagiotakos, D.B. Whole Grain Consumption and Breast Cancer: A Case-Control Study in Women. J. Am. Coll. Nutr. 2016, 35, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Mouw, T.; Park, Y.; Subar, A.F.; Kipnis, V.; Hollenbeck, A.; Leitzmann, M.F.; Thompson, F.E. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2007, 85, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Tighe, P.; Duthie, G.; Vaughan, N.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Mutch, W.; Wahle, K.; Horgan, G.; Thies, F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 92, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tao, G.; Liu, P.; Liu, J. Peptide with Angiotensin I-Converting Enzyme Inhibitory Activity from Hydrolyzed Corn Gluten Meal. J. Agric. Food Chem. 2007, 55, 7891–7895. [Google Scholar] [CrossRef] [PubMed]
- Duru, C.E. Mineral and phytochemical evaluation of Zea mays husk. Sci. Afr. 2020, 7, e00224. [Google Scholar] [CrossRef]
- Luthria, D.L.; Lu, Y.; John, K.M.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. (Eds.) Chapter 6—Nutritional value of wheat. In Wheat—An Exceptional Crop; Woodhead Publishing: Sawston, UK, 2020; pp. 133–148. ISBN 978-0-12-821715-3. [Google Scholar]
- Kim, S.-K.; Ngo, D.-H.; Vo, T.-S. Chapter 16—Marine Fish-Derived Bioactive Peptides as Potential Antihypertensive Agents. In Marine Medicinal Foods; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, pp. 249–260. ISBN 1043-4526. [Google Scholar]
- Barbosa, J.R.; de Carvalho Junior, R.N. Occurrence and possible roles of polysaccharides in fungi and their influence on the development of new technologies. Carbohydr. Polym. 2020, 246, 116613. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.; Shang, J.; Chen, J.; Xu, X. Dectin-1 mediates the immunoenhancement effect of the polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2018, 109, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, G.; You, L.; Zhang, L.; Ren, H.; Hu, W.; Qiang, Q.; Wang, X.; Ji, L.; Gu, Z.; et al. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (Oat), a potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef]
- Soycan, G.; Schär, M.Y.; Kristek, A.; Boberska, J.; Alsharif, S.N.S.; Corona, G.; Shewry, P.R.; Spencer, J.P.E. Composition and content of phenolic acids and avenanthramides in commercial oat products: Are oats an important polyphenol source for consumers? Food Chem. X 2019, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Adam Itodo, O.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Fardet, A.; Tosh, S.M.; Rich, G.T.; Wilde, P.J. Processing of oat: The impact on oat’s cholesterol lowering effect. Food Funct. 2018, 9, 1328–1343. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, L.; Huang, L.; Shi, Z.; Dong, J.; Yao, Y.; Shen, R. Effects of oat β-glucan, oat resistant starch, and the whole oat flour on insulin resistance, inflammation, and gut microbiota in high-fat-diet-induced type 2 diabetic rats. J. Funct. Foods 2020, 69, 103939. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Investigation of phenolic compounds with antioxidant activity in barley and oats affected by variation in growing location. Cereal Chem. 2020, 97, 772–782. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Alashi, A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Purification and hypotensive activity of rapeseed protein-derived renin and angiotensin converting enzyme inhibitory peptides. J. Funct. Foods 2013, 5, 781–789. [Google Scholar] [CrossRef]
- Dong, J.; Yang, M.; Zhu, Y.; Shen, R.; Zhang, K. Comparative study of thermal processing on the physicochemical properties and prebiotic effects of the oat β-glucan by in vitro human fecal microbiota fermentation. Food Res. Int. 2020, 138, 109818. [Google Scholar] [CrossRef]
- Pino, J.L.; Mujica, V.; Arredondo, M. Effect of dietary supplementation with oat β-glucan for 3 months in subjects with type 2 diabetes: A randomized, double-blind, controlled clinical trial. J. Funct. Foods 2021, 77, 104311. [Google Scholar] [CrossRef]
- Maki, K.C.; Galant, R.; Samuel, P.; Tesser, J.; Witchger, M.S.; Ribaya-Mercado, J.D.; Blumberg, J.B.; Geohas, J. Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur. J. Clin. Nutr. 2007, 61, 786–795. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020, 63, 383–385. [Google Scholar] [CrossRef]
- Duodu, K.G.; Awika, J.M. Chapter 8—Phytochemical-Related Health-Promoting Attributes of Sorghum and Millets. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 225–258. ISBN 978-0-12-811527-5. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Choi, Y.-Y.; Osada, K.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci. Biotechnol. Biochem. 2005, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Li, Z.; Newton, I.P.; Zhao, C.; Li, Z.; Guo, M. A novel protein extracted from foxtail millet bran displays anti-carcinogenic effects in human colon cancer cells. Toxicol. Lett. 2014, 227, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Panahi, S.; Kaufman, S.; Wu, J. Fried egg digest decreases blood pressure in spontaneous hypertensive rats. J. Funct. Foods 2013, 5, 187–194. [Google Scholar] [CrossRef]
- Baksi, A.J.; Treibel, T.A.; Davies, J.E.; Hadjiloizou, N.; Foale, R.A.; Parker, K.H.; Francis, D.P.; Mayet, J.; Hughes, A.D. A meta-analysis of the mechanism of blood pressure change with aging. J. Am. Coll. Cardiol. 2009, 54, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.S. Functional properties of cereal cell wall polysaccharides. In Carbohydrates in Food; CRC Press: Boca Raton, FL, USA, 2017; pp. 215–278. ISBN 978-1-315-37282-2. [Google Scholar]
- Pihlava, J.-M.; Hellström, J.; Kurtelius, T.; Mattila, P. Flavonoids, anthocyanins, phenolamides, benzoxazinoids, lignans and alkylresorcinols in rye (Secale cereale) and some rye products. J. Cereal Sci. 2018, 79, 183–192. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, I.; Sharma, M.; Mehta, M.; Badyal, S.; Sharma, V.; Sharma, I.; Singh, H.; Sistla, S. Bioactive compounds and probiotics–a ray of hope in COVID-19 management. Food Sci. Hum. Wellness 2021, 10, 131–140. [Google Scholar] [CrossRef]
- Wouk, J.; Dekker, R.F.H.; Queiroz, E.A.I.F.; Barbosa-Dekker, A.M. β-Glucans as a panacea for a healthy heart? Their roles in preventing and treating cardiovascular diseases. Int. J. Biol. Macromol. 2021, 177, 176–203. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Althwab, S.; Carr, T.P.; Weller, C.L.; Dweikat, I.M.; Schlegel, V. Advances in grain sorghum and its co-products as a human health promoting dietary system. Food Res. Int. 2015, 77, 349–359. [Google Scholar] [CrossRef]
- Chiremba, C.; Taylor, J.R.N.; Rooney, L.W.; Beta, T. Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chem. 2012, 134, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthoycanins from black sorghum and their antioxidant properties. Food Chem. 2004, 90, 293–301. [Google Scholar] [CrossRef]
- Bean, S.R.; Wilson, J.D.; Moreau, R.A.; Galant, A.; Awika, J.M.; Kaufman, R.C.; Adrianos, S.L.; Ioerger, B.P. Structure and Composition of the Sorghum Grain. Sorghum 2019, 58, 173–214. [Google Scholar] [CrossRef]
- Bhandari, S.; Lee, Y.-S. The Contents of Phytosterols, Squalene, and Vitamin E and the Composition of Fatty Acids of Korean Landrace Setaria italica and Sorghum bicolar Seeds. Korean J. Plant Resour. 2013, 26, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]



| Food | Main Phenolic Compound | Test | IC50 or % IECA | Decrease BP | Main Mechanism | Reference |
|---|---|---|---|---|---|---|
| Virgin rice bran oil | Sterols, tocopherols, and tocotrienols | in vivo | ND | 25.5% | Regulation of NOS and reduction in oxidative stress | [54] |
| Raw rice | Phenol acids Flavonoids | in vitro | 97% | ND | Competitive inhibition of ECA | [55] |
| Rice bran hydrolysate | Phenolic compounds | in vivo | ND | 31.5% | Endothelium-derived hyperpolarizing factor-mediated vasorelaxation and L-type Ca 2+ channel-mediated vasoconstriction | [56] |
| Barley seedlings | Polyphenols | in vitro | 66.5% | ND | Non-competitive inhibitors of ECA and formation of chelates with ions of zinc | [57] |
| Barley whole grain | Anthocyanins | in vitro | 8770 µg/mL | ND | Natural competitive inhibitors of ECA | [58] |
| Barley bran | 4540 µg/mL | |||||
| Solid-state fermented wheat | Phenolic compounds | in vitro | 53.8% | ND | Inhibition of ECA by proteolysis | [59] |
| Bioprocessed wheat middlings | Phenolic compounds | in vitro | 94.9% | ND | The hydrolysis of short chain peptides increases ECA- inhibitory capacity | [60] |
| Sorghum roasted grain | Phenolic acids and flavonoids | in vitro | 20.99 µg/mL | ND | Hydrogen and the hydrophobic union caused by the denaturation of enzymes | [61] |
| Sorghum grains | Phenolic compounds | in vitro | 46.3% | ND | Production of peptides and free amino acids before germination increases ECA-inhibitory activity | [62] |
| Extruded maize products added with a red seaweed | Phenolic compounds | in vitro | 41% | ND | ECA inhibition trough sequestration of enzyme metal factor Zn2+ | [53] |
| Water extracts of maize | Soluble phenols | in vitro | 50% | ND | Small peptide compounds may represent the bioactive factors contributing to the total ECA-inhibitory activity | [63] |
| Food | Bioactive Compound | MW | Test | IC50 or % IECA | Decrease BP | Main Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Bran of rice | Peptide | <4 kDa | in vitro | 30 µg/mL | ND | Reducer and inhibitor of ECA | [67] |
| Rice protein hydrolysates | Dipeptides | ND | in vitro | 76.58-µg/mL | ND | Blocker of ECA due to the presence of aromatic amino acids | [72] |
| Barley flour | Peptide | <3 kDa | in vitro | 70.3% | ND | Inhibitors of ECA via the presence of hydrophobic amino acids | [68] |
| Corn germ flour | Peptide | <3 kDa | in vivo | 830 µg/mL | 15.7% | Regulation of vasoconstrictors increases in NO and prostacyclin decreases in Ang II | [73] |
| Corn germ | Peptides | <6 kDa | in vitro | 1389 µg/mL | ND | Inhibitory effect on ECA | [74] |
| Corn gluten flour | Peptides | <3 kDa | in vivo/in vitro | 290 µg/mL | >30 mmHg SBP | Persistent inhibition of the ECA in tissues | [75] |
| Corn gluten flour | Dipeptide | ND | in vivo-in vitro | 37 µg/mL | 35–45 mmHg SBP | Inhibitor of ECA by possible synergy between peptides | [76] |
| Hydrolyzed wheat gluten | Peptides | <1 kDa | in vitro | 2 µg/mL | ND | Inhibition of ECA by electrostatic interactions and interactions with hydrogen bonds | [66] |
| Hydrolyzed wheat gluten | Peptides | <1 kDa | in vitro | 4 µg/mL | ND | Competitive and non-competitive inhibitors of ECA | [77] |
| Defatted wheat germ | Peptides | <5 kDa | in vitro | 452 µg/mL | ND | Inhibition of ECA by enzymolysis and ionization of proteins | [78] |
| Defatted wheat germ | Hydrolyzed proteins | ND | in vitro | 220 µg/mL | ND | Inhibition of ECA by hydrophobic amino acids | [79] |
| Wheat flour | Phenolics from peptide fractions | ND | in vitro | 84.52% | ND | Inhibition of ECA by bound phenols after acid hydrolysis | [80] |
| Oat-isolated protein | Peptides | <3 kDa | in vitro | 60% | ND | Ultrasonic pretreated enzymolisis increased ECA-inhibitory activities of the oat peptides | [81] |
| Oat protein hydrolysate | Peptides | ND | in silico | 96.5% | ND | Inhibition of ECA-I by aromatic, small acids with low lipophilicity and high electronic properties | [82] |
| Oat protein hydrolysate | Peptides | <3 kDa | in vitro e in silico | 35 µg/mL | ND | Competitive inhibitors of ECA | [83] |
| Sweet sorghum grain | Peptides fractions | <1 kDa | in vitro | 31.6 µg/mL | ND | Binding of the C-terminal of Serine with the active sites of ECA | [84] |
| Sorghum protein hydrolysate | Tripeptides | ND | in vitro | 1.3 µg/mL | ND | Competitive inhibitor of ECA | [85] |
| Bread produced with addition of 6% rye-malt gluten | Peptides | ND | in vitro | 0.002 µM/mL | ND | ECA binding at the N-terminal and proline or aromatic amino acids at the C-terminus | [86] |
| Extruded and fermented millet | Peptides | ND | in vivo | ND | 14.6% | Reduction in the indexes of RAAS | [87] |
| Bread or sandwiches with pure millet grains | Protein | ND | Clinical | ND | 3% | Inhibition of vasoconstrictors and induction of vasodilators | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Castro, A.; Román-Gutiérrez, A.D.; Castañeda-Ovando, A.; Cariño-Cortés, R.; Acevedo-Sandoval, O.A.; López-Perea, P.; Guzmán-Ortiz, F.A. Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19. Foods 2022, 11, 3231. https://doi.org/10.3390/foods11203231
García-Castro A, Román-Gutiérrez AD, Castañeda-Ovando A, Cariño-Cortés R, Acevedo-Sandoval OA, López-Perea P, Guzmán-Ortiz FA. Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19. Foods. 2022; 11(20):3231. https://doi.org/10.3390/foods11203231
Chicago/Turabian StyleGarcía-Castro, Abigail, Alma Delia Román-Gutiérrez, Araceli Castañeda-Ovando, Raquel Cariño-Cortés, Otilio Arturo Acevedo-Sandoval, Patricia López-Perea, and Fabiola Araceli Guzmán-Ortiz. 2022. "Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19" Foods 11, no. 20: 3231. https://doi.org/10.3390/foods11203231
APA StyleGarcía-Castro, A., Román-Gutiérrez, A. D., Castañeda-Ovando, A., Cariño-Cortés, R., Acevedo-Sandoval, O. A., López-Perea, P., & Guzmán-Ortiz, F. A. (2022). Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19. Foods, 11(20), 3231. https://doi.org/10.3390/foods11203231