Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
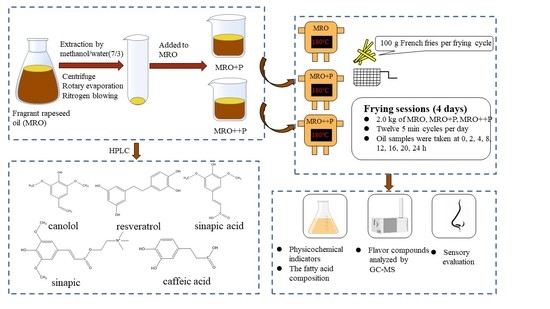
2.2.1. Extraction of Phenolic Compounds
2.2.2. Oil Samples Preparation
2.2.3. Simulated Frying Process
2.2.4. Determination of Peroxide Value (PV), Acide Value (AV), p-Anisidine (p-AnV), Carbonyl Value (CV), Total Popar Compound Content (TPC)
2.2.5. Determination of Phenolic Compounds
2.2.6. Determination of Fatty Acid Composition
2.2.7. Determination of Flavor Compounds
2.2.8. Evaluation Method of Main Flavor Compounds
2.2.9. Sensory Evaluation
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Oil Samples
3.2. Effects of HCP on Physicochemical Indicators of Frying Oil
3.3. Effects of HCP on Fatty Acids Composition
3.4. Effects of HCP on Flavor Compounds
3.5. Effects of HCP on Characteristic Flavor Compounds
3.6. Effects of HCP on Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhu, Y.; Shi, L.; Guo, Y.; Wei, L.; Zhang, H.; Wang, X.; Jin, Q. Physicochemical properties and health risk assessment of polycyclic aromatic hydrocarbons of fragrant rapeseed oils in China. J. Sci. Food Agric. 2020, 100, 3351–3359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jia, X.; Yao, Y.Z.; Wang, B.; Wei, C.Q.; Zhang, M.; Huang, F. Characterization of the Aroma-Active Compounds in Commercial Fragrant Rapeseed Oils via Monolithic Material Sorptive Extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Wang, J.; Pan, Q.; Luo, Y.; Sun, Y.; Jin, Q.; Wang, X. Assessment of contamination source and quality control approach for polycyclic aromatic hydrocarbons in wood-pressed rapeseed oil. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, M.; Ramadan, M.F. Volatile Oxidation Compounds and Stability of Safflower, Sesame and Canola Cold-Pressed Oils as Affected by Thermal and Microwave Treatments. J. Oleo Sci. 2016, 65, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Tynek, M.; Pawłowicz, R.; Gromadzka, J.; Tylingo, R.; Wardencki, W.; Karlovits, G. Virgin rapeseed oils obtained from different rape varieties by cold pressed method–Their characteristics, properties, and differences. Eur. J. Lipid Sci. Technol. 2012, 114, 357–366. [Google Scholar] [CrossRef]
- Wei, F.; Yang, M.; Zhou, Q.; Zheng, C.; Peng, J.-H.; Liu, C.-S.; Huang, F.-H.; Chen, H. Varietal and processing effects on the volatile profile of rapeseed oils. LWT-Food Sci. Technol. 2012, 48, 323–329. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases- A critical review. Trends Food Sci. Technol. 2018, 71, 132–154. [Google Scholar] [CrossRef]
- Roszkowska, B.; Tańska, M.; Czaplicki, S.; Konopka, I. Variation in the composition and oxidative stability of commercial rapeseed oils during their shelf life. Eur. J. Lipid Sci. Technol. 2014, 117, 673–683. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Chang, C.; Lv, Y.; Lai, W.; Zhang, H.; Wang, X.; Jin, Q. Determination of Origin of Commercial Flavored Rapeseed Oil by the Pattern of Volatile Compounds Obtained via GC–MS and Flash GC Electronic Nose. Eur. J. Lipid Sci. Technol. 2020, 122, 1900332. [Google Scholar] [CrossRef]
- Sordini, B.; Veneziani, G.; Servili, M.; Esposto, S.; Selvaggini, R.; Lorefice, A.; Taticchi, A. A quanti-qualitative study of a phenolic extract as a natural antioxidant in the frying processes. Food Chem. 2019, 279, 426–434. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H.; Mildner-Szkudlarz, S.; Jasińska, I.; Wąsowicz, E. A Headspace–SPME–MS Method for Monitoring Rapeseed Oil Autoxidation. J. Am. Oil Chem. Soc. 2007, 84, 509–517. [Google Scholar] [CrossRef]
- Sghaier, L.; Cordella, C.B.Y.; Rutledge, D.N.; Watiez, M.; Breton, S.; Kopczuk, A.; Sassiat, P.; Thiebaut, D.; Vial, J. Comprehensive Two-dimensional Gas Chromatography for Analysis of the Volatile Compounds and Fishy Odor Off-flavors from Heated Rapeseed Oil. Chromatographia 2015, 78, 805–817. [Google Scholar] [CrossRef]
- Xu, L.; Mei, X.; Chang, J.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Comparative characterization of key odorants of French fries and oils at the break-in, optimum, and degrading frying stages. Food Chem. 2022, 368, 130581. [Google Scholar] [CrossRef] [PubMed]
- Thiyam, U.; Claudia, P.; Jan, U.; Alfred, B. De-oiled rapeseed and a protein isolate: Characterization of sinapic acid derivatives by HPLC–DAD and LC–MS. Eur. Food Res. Technol. 2009, 229, 825–831. [Google Scholar] [CrossRef]
- Han, L.; Xia, X.; Xiang, X.; Huang, F.; Zhang, Z. Protective effects of canolol against hydrogen peroxide-induced oxidative stress in AGS cells. RSC Adv. 2017, 7, 42826–42832. [Google Scholar] [CrossRef] [Green Version]
- Alu’datt, M.H.; Rababah, T.; Alli, I. Effect of phenolic compound removal on rheological, thermal and physico-chemical properties of soybean and flaxseed proteins. Food Chem. 2014, 146, 608–613. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Dar, M.M. Effect of apple pomace-based antioxidants on the stability of mustard oil during deep frying of French fries. LWT 2022, 163, 113576. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Rudzinska, M.; Siger, A.; Gramza-Michalowska, A.; Kobus-Cisowska, J. Thermo-Oxidation of Phytosterol Molecules in Rapeseed Oil during Heating: The Impact of Unsaturation Level of the Oil. Foods 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mei, X.; Wu, G.; Karrar, E.; Jin, Q.; Wang, X. Inhibitory effect of antioxidants on key off-odors in French fries and oils and prolong the optimum frying stage. LWT 2022, 162, 113431. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Model for prediction of the carbonyl value of frying oil from the initial composition. LWT 2020, 117, 108660. [Google Scholar] [CrossRef]
- Guan, M.; Chen, H.; Xiong, X.; Lu, X.; Li, X.; Huang, F.; Guan, C. A Study on Triacylglycerol Composition and the Structure of High-Oleic Rapeseed Oil. Engineering 2016, 2, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Pang, M.; Cao, L.; Kang, S.; Jiang, S.; Cao, L. Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods 2021, 10, 1828. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Han, B.; Wu, W.; Zhao, Q.; Wei, C.; Liu, W. Comprehensive analysis of volatile compounds in cold-pressed safflower seed oil from Xinjiang, China. Food Sci. Nutr. 2020, 8, 903–914. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, C.; Wang, C.; Chen, H.; Liu, Y.; Li, S.; Lin, D.; Wu, D.; Qin, W. Evaluation of the non-aldehyde volatile compounds formed during deep-fat frying process. Food Chem. 2018, 243, 151–161. [Google Scholar] [CrossRef]
- Kraljic, K.; Skevin, D.; Barisic, L.; Kovacevic, M.; Obranovic, M.; Jurcevic, I. Changes in 4-vinylsyringol and other phenolics during rapeseed oil refining. Food Chem. 2015, 187, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; Yu, J.; Chen, N.; Zeng, W.C. Effects and mechanism of tea polyphenols on the quality of oil during frying process. J. Food Sci. 2020, 85, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.; Rudzińska, M.; Przybylski, R.; Kmiecik, D.; Siger, A.; Olejnik, A. The degradation of bioactive compounds and formation of their oxidation derivatives in refined rapeseed oil during heating in model system. LWT 2020, 123, 109278. [Google Scholar] [CrossRef]
- Aniolowska, M.; Zahran, H.; Kita, A. The effect of pan frying on thermooxidative stability of refined rapeseed oil and professional blend. J. Food Sci. Technol. 2016, 53, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Shrestha, K.; Gemechu, F.G.; De Meulenaer, B. A novel insight on the high oxidative stability of roasted mustard seed oil in relation to phospholipid, Maillard type reaction products, tocopherol and canolol contents. Food Res. Int. 2013, 54, 587–594. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, S.; Yang, B.; Zhang, H.; Wang, X.; Granvogl, M.; Jin, Q. Flavor of rapeseed oil: An overview of odorants, analytical techniques, and impact of treatment. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3983–4018. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.; Shen, Q.; Saleh, A.S. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015, 52, 7683–7696. [Google Scholar] [CrossRef]




| MRO | MRO+P | MRO++P | |
|---|---|---|---|
| Sinapine | 5.81 ± 0.52 c | 7.52 ± 0.43 b | 11.77 ± 0.56 a |
| Caffeic acid | 3.63 ± 0.41 c | 4.70 ± 0.32 b | 7.36 ± 0.43 a |
| Resveratrol | 3.27 ± 0.35 c | 4.23 ± 0.41 b | 6.62 ± 0.42 a |
| Sinapic acid | 14.52 ± 0.87 c | 18.79 ± 1.12 b | 29.42 ± 0.97 a |
| Canolol | 154.32 ± 3.54 c | 234.89 ± 4.01 b | 312.61 ± 4.86 a |
| Total phenol content (HPLC) | 181.48 ± 3.79 c | 276.34 ± 4.50 b | 367.77 ± 5.22 a |
| Fatty Acids | Fatty Acids Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | C22:1 | SFA | MUFA | ||
| 0 h | MRO | 6.82 ± 0.02 Da | 2.87 ± 0.02 Ca | 49.95 ± 0.22 Ca | 30.5 ± 0.19 Aa | 8.15 ± 0.04 Ab | 0.92 ± 0 Bb | 0.8 ± 0.02 Aa | 9.69 ± 0.03 Da | 90.31 ± 0.03 Aa |
| MRO+P | 6.85 ± 0.02 Da | 2.67 ± 0.03 Db | 50.44 ± 0.23 Ca | 30.21 ± 0.25 Aa | 8.25 ± 0.03 Aa | 0.91 ± 0.01 ABb | 0.68 ± 0.02 Bb | 9.51 ± 0.04 Db | 90.49 ± 0.04 Aa | |
| MRO++P | 6.78 ± 0.08 Da | 2.65 ± 0.01 Db | 50.47 ± 0.2 Da | 30.25 ± 0.22 Aa | 8.31 ± 0.01 Aa | 0.95 ± 0.01 Aa | 0.6 ± 0.02 Ac | 9.43 ± 0.08 Db | 90.57 ± 0.08 Aa | |
| 8 h | MRO | 8.77 ± 0.06 Ca | 2.87 ± 0.02 Ca | 53.26 ± 0.12 Aa | 26.2 ± 0.06 Bc | 7.22 ± 0.06 Ba | 0.93 ± 0.01 Ba | 0.74 ± 0.01 Ba | 11.65 ± 0.04 Cab | 88.35 ± 0.04 Bab |
| MRO+P | 8.79 ± 0.04 Ca | 2.94 ± 0.03 Cb | 52.29 ± 0.11 Bb | 27.56 ± 0.06 Bb | 6.98 ± 0.1 Bb | 0.91 ± 0.02 ABa | 0.53 ± 0 Cc | 11.73 ± 0.07 Ca | 88.27 ± 0.07 Bb | |
| MRO++P | 8.55 ± 0.12 Cb | 2.93 ± 0.03 Cb | 51.79 ± 0.15 Cc | 28.2 ± 0.08 Ba | 7.06 ± 0.04 Bab | 0.92 ± 0.01 Ba | 0.55 ± 0.01 Bb | 11.48 ± 0.14 Cb | 88.52 ± 0.14 Ba | |
| 16 h | MRO | 13.74 ± 0.15 Ba | 3.14 ± 0.04 Ba | 51.66 ± 0.33 Bc | 23.67 ± 0.22 Cc | 6.13 ± 0.06 Ca | 0.96 ± 0.01 Aa | 0.7 ± 0.01 Cb | 16.88 ± 0.18 Ba | 83.12 ± 0.18 Cc |
| MRO+P | 12.31 ± 0.07 Bb | 3.15 ± 0.01 Ba | 52.96 ± 0.2 Ab | 24.21 ± 0.22 Cb | 5.61 ± 0.04 Cb | 0.96 ± 0.04 Aa | 0.8 ± 0.04 Aa | 15.47 ± 0.06 Bb | 84.53 ± 0.06 Cb | |
| MRO++P | 11.26 ± 0.04 Bc | 3.10 ± 0.04 Ba | 53.75 ± 0.14 Aa | 25.06 ± 0.06 Ca | 5.41 ± 0.04 Cc | 0.9 ± 0.01 Cb | 0.52 ± 0.01 Cc | 14.37 ± 0.07 Bc | 85.63 ± 0.07 Ca | |
| 24 h | MRO | 15.87 ± 0.14 Aa | 3.28 ± 0.02 Aa | 51.72 ± 0.49 Bb | 22.46 ± 0.36 Db | 5.08 ± 0.03 Db | 0.95 ± 0.01 Aa | 0.64 ± 0.02 Db | 19.15 ± 0.17 Aa | 80.85 ± 0.17 Dc |
| MRO+P | 14.52 ± 0.31 Ab | 3.51 ± 0.03 Ab | 51.97 ± 0.44 Bb | 22.78 ± 0.16 Dab | 5.56 ± 0.13 Ca | 0.89 ± 0.02 Bb | 0.77 ± 0.02 Aa | 18.03 ± 0.27 Ab | 81.97 ± 0.27 Db | |
| MRO++P | 13.83 ± 0.1 Ac | 3.58 ± 0.05 Ab | 53.28 ± 0.07 Ba | 23.34 ± 0.1 Da | 4.56 ± 0.04 Dc | 0.88 ± 0 Db | 0.54 ± 0 BCc | 17.41 ± 0.12 Ac | 82.59 ± 0.12 Da | |
| Compounds | The Number and Relative Content of Flavor Compounds | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 8 h | 16 h | 24 h | |||||||||||||||||||||
| MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | |||||||||||||
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| alcohols | 13 | 7.38 | 13 | 7.51 | 13 | 7.45 | 9 | 5.96 | 10 | 5.89 | 11 | 5.79 | 8 | 5.54 | 8 | 5.61 | 10 | 5.58 | 6 | 4.01 | 6 | 4.13 | 9 | 4.11 |
| aldehydes | 13 | 17.4 | 12 | 16.9 | 12 | 17.4 | 19 | 74.48 | 20 | 70.11 | 18 | 69.08 | 18 | 77.95 | 19 | 74.38 | 20 | 72.04 | 21 | 82.28 | 18 | 76.84 | 21 | 74.01 |
| acids | 7 | 3.08 | 7 | 3.1 | 8 | 3.11 | 3 | 4.67 | 5 | 4.41 | 6 | 4.03 | 4 | 5.49 | 3 | 5.23 | 4 | 4.93 | 4 | 6.36 | 4 | 5.87 | 5 | 5.29 |
| ketones | 9 | 17.8 | 9 | 16.9 | 10 | 16.6 | 1 | 4.28 | 3 | 6.38 | 6 | 7.98 | 1 | 3.11 | 2 | 3.27 | 5 | 4.02 | 1 | 1.92 | 1 | 1.99 | 3 | 2.43 |
| esters | 5 | 0.08 | 4 | 0.14 | 5 | 0.15 | ND | ND | 1 | 0.11 | 1 | 0.12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| hydrocarbons | 9 | 2.95 | 10 | 3.01 | 9 | 3.21 | 5 | 1.39 | 7 | 1.37 | 7 | 1.41 | 4 | 0.87 | 6 | 0.88 | 7 | 0.91 | 2 | 0.81 | 5 | 0.79 | 6 | 0.83 |
| heterocycles | 15 | 25.84 | 15 | 23.91 | 12 | 24.53 | 7 | 8.07 | 9 | 10.04 | 9 | 9.66 | 8 | 6.48 | 10 | 9.96 | 9 | 11.49 | 6 | 4.73 | 11 | 9.94 | 9 | 12.43 |
| thiolaside degradation products | 15 | 25.47 | 16 | 28.53 | 13 | 27.55 | 3 | 2.25 | 4 | 2.59 | 6 | 2.53 | 1 | 1.56 | 2 | 1.67 | 5 | 1.63 | 1 | 1.39 | 2 | 1.44 | 3 | 1.4 |
| Compounds | Concentration (μg/g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odor Description | ROAV | 0 h | 8 h | 16 h | 24 h | ||||||||||||
| 0 h | 8 h | 16 h | 24 h | MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | MRO | MRO+P | MRO++P | ||
| Hexanoic acid | sweaty | 1.59 | 0.69 | 0.59 | 0.63 | 0.08 ± 0.03 Da | 0.08 ± 0.02 Da | 0.08 ± 0.02 Da | 1.22 ± 0.04 Ca | 1.03 ± 0.05 Cb | 0.79 ± 0.03 Cc | 3.08 ± 0.09 Ba | 2.42 ± 0.06 Bb | 2.05 ± 0.07 Bc | 5.08 ± 0.09 Aa | 3.82 ± 0.06 Ab | 2.45 ± 0.07 Ac |
| Octanoic acid | sweat, cheese, rancid | 2.51 | 2.03 | 2.12 | 1.89 | 0.16 ± 0.04 Da | 0.15 ± 0.03 Da | 0.16 ± 0.04 Da | 1.06 ± 0.05 Ca | 0.87 ± 0.06 Cb | 0.67 ± 0.03 Cc | 1.42 ± 0.04 Ba | 1.24 ± 0.06 Bb | 0.95 ± 0.05 Bc | 2.25 ± 0.03 Aa | 1.92 ± 0.05 Ab | 1.21 ± 0.07 Ac |
| Nonanoic acid | green, fat, rancid | 1.44 | 1.35 | 1.09 | 1.11 | 0.25 ± 0.06 Da | 0.25 ± 0.04 Da | 0.26 ± 0.05 Da | 0.98 ± 0.04 Ca | 0.75 ± 0.03 Cb | 0.62 ± 0.04 Cc | 1.19 ± 0.05 Ba | 1.02 ± 0.04 Bb | 0.75 ± 0.04 Bc | 2.04 ± 0.05 Aa | 1.45 ± 0.03 Ab | 0.98 ± 0.04 Ac |
| Hexanal | fat, citrus, rancid | 1.01 | 0.90 | 1.62 | 1.70 | 0.78 ± 0.03 Da | 0.82 ± 0.05 Da | 0.86 ± 0.05 Da | 6.60 ± 0.05 Ca | 4.9 ± 0.05 Cb | 2.95 ± 0.06 Cc | 9.09 ± 0.04 Ba | 7.62 ± 0.06 Bb | 5.87 ± 0.07 Bc | 11.18 ± 0.33 Aa | 9.29 ± 0.13 Ab | 6.42 ± 0.06 Ac |
| (E)-2-Heptenal | soap, fat, almond | 7.33 | 67.16 | 72.37 | 73.49 | 0.32 ± 0.04 Da | 0.35 ± 0.05 Da | 0.28 ± 0.02 Da | 9.78 ± 0.05 Ca | 7.32 ± 0.15 Cb | 5.81 ± 0.12 Cc | 13.23 ± 0.24 Aa | 11.90 ± 0.08 Ab | 10.12 ± 0.1 Ac | 10.8 ± 0.1 Ba | 9.54 ± 0.13 Bb | 8.08 ± 0.05 Bc |
| 1-Heptanol | chemical, green | ND | 1.30 | 3.25 | 3.31 | ND | ND | ND | 0.65 ± 0.03 Ba | 0.49 ± 0.05 Bb | 0.30 ± 0.02 Bc | 0.81 ± 0.05 Aa | 0.63 ± 0.02 Ab | 0.32 ± 0.02 Bc | 0.37 ± 0.03 Cb | 0.41 ± 0.02 Cb | 1.04 ± 0.02 Aa |
| 1-Octen-3-ol | mushroom | ND | 100 | 100 | 100 | ND | ND | ND | 2.06 ± 0.06 Ba | 1.57 ± 0.06 Cb | 1.37 ± 0.05 Cc | 2.05 ± 0.04 Ba | 1.85 ± 0.02 Bb | 1.71 ± 0.03 Bc | 3.07 ± 0.1 Aa | 2.84 ± 0.12 Aa | 2.54 ± 0.08 Ab |
| Dimethyl trisulfide | sulfur, fish, cabbage | 5.72 | ND | ND | ND | 0.17 ± 0 Aa | 0.18 ± 0 Aa | 0.17 ± 0.01 Aa | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2-Pentylfuran | green bean, butter | 1.32 | 1.79 | 2.26 | 1.95 | 0.99 ± 0.03 Ca | 0.96 ± 0.02 Da | 1.01 ± 0.03 Da | 3.19 ± 0.04 Ba | 2.75 ± 0.04 Cb | 1.83 ± 0.06 Cc | 3.61 ± 0.07 Aa | 3.18 ± 0.13 Bb | 2.28 ± 0.07 Bc | 3.73 ± 0.13 Aa | 3.59 ± 0.04 Ab | 2.69 ± 0.04 Ac |
| (E)-2-Octenal | green, nut, fat | 8.81 | 57.32 | 59.24 | 60.78 | 0.36 ± 0.02 Da | 0.34 ± 0.03 Da | 0.31 ± 0.02 Da | 5.8 ± 0.19 Ca | 5.17 ± 0.1 Cb | 4.71 ± 0.09 Cc | 11.91 ± 0.17 Aa | 10.41 ± 0.29 Ab | 9.07 ± 0.04 Ac | 7.46 ± 0.06 Ba | 6.41 ± 0.09 Bb | 6.29 ± 0.08 Bc |
| (E,E)-2,4-Heptadienal | nut, fat | 2.58 | 2.93 | 6.91 | 5.91 | 1.01 ± 0.03 Da | 0.97 ± 0.06 Da | 1.06 ± 0.05 Ca | 28.58 ± 0.38 Aa | 27.91 ± 0.23 Ab | 25.95 ± 0.71 Ab | 25.79 ± 0.24 Ca | 24.32 ± 0.2 Cb | 22.32 ± 0.81 Bc | 26.84 ± 0.13 Ba | 24.93 ± 0.06 Bb | 22.29 ± 0.88 Bc |
| 1-Octanol | chemical, metal, burnt | 3.04 | 1.20 | 1.52 | 1.97 | 0.43 ± 0.01 Da | 0.44 ± 0.03 Da | 0.48 ± 0.04 Da | 1.39 ± 0.03 Ca | 0.81 ± 0.03 Cb | 0.64 ± 0.04 Cc | 1.57 ± 0.05 Ba | 1.22 ± 0.02 Bb | 1.06 ± 0.03 Bc | 1.76 ± 0.07 Aa | 1.58 ± 0.02 Ab | 1.44 ± 0.02 Ac |
| Nonanal | fat, citrus, green | 1.31 | 2.47 | 2.95 | 3.20 | 1.45 ± 0.01 Da | 1.35 ± 0.09 Ca | 1.32 ± 0.04 Da | 18.93 ± 0.39 Ca | 12.94 ± 0.07 Bb | 8.33 ± 0.15 Cc | 22.52 ± 0.44 Ba | 13.83 ± 0.55 Bb | 12.71 ± 0.13 Bc | 29.86 ± 0.31 Aa | 24.23 ± 0.82 Ab | 19.43 ± 0.26 Ac |
| (E,E)-2,4-Nonadienal | fat, wax, green | ND | 13.52 | 12.52 | 12.67 | ND | ND | ND | 0.86 ± 0.02 Ca | 0.72 ± 0.01 Cb | 0.62 ± 0.02 Cc | 1.59 ± 0.04 Aa | 1.49 ± 0.04 Ab | 1.07 ± 0.04 Ac | 1.41 ± 0.04 Ba | 1.32 ± 0.05 Ba | 0.97 ± 0.02 Bb |
| (E)-2-Decenal | tallow | 2.67 | 4.87 | 4.39 | 4.11 | 0.73 ± 0.01 Da | 0.7 ± 0.04 Da | 0.71 ± 0.03 Da | 10.65 ± 0.11 Ca | 8.88 ± 0.09 Cb | 7.34 ± 0.16 Cc | 14.59 ± 0.31 Aa | 13.26 ± 0.58 Ab | 11.44 ± 0.12 Ac | 13.51 ± 0.33 Ba | 11.55 ± 0.31 Bb | 10.19 ± 0.08 Bc |
| (E,E)-2,4-Decadienal | deep-fried, wax, fat | 1.71 | 2.69 | 1.94 | 1.58 | 3.71 ± 0.07 Da | 3.71 ± 0.11 Da | 3.69 ± 0.09 Ca | 22.16 ± 0.69 Bc | 24.42 ± 0.3 Cb | 25.9 ± 0.12 Ca | 23.67 ± 0.18 Ac | 28.98 ± 0.34 Bb | 31.56 ± 0.3 Ba | 20.19 ± 0.04 Bc | 25.13 ± 0.76 Ab | 27.86 ± 0.68 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Jia, P.; Liu, H.; Kang, S.; Jiang, S.; Pang, M. Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying. Foods 2023, 12, 827. https://doi.org/10.3390/foods12040827
Cao L, Jia P, Liu H, Kang S, Jiang S, Pang M. Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying. Foods. 2023; 12(4):827. https://doi.org/10.3390/foods12040827
Chicago/Turabian StyleCao, Lili, Pengpeng Jia, Haotian Liu, Shengmei Kang, Shaotong Jiang, and Min Pang. 2023. "Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying" Foods 12, no. 4: 827. https://doi.org/10.3390/foods12040827
APA StyleCao, L., Jia, P., Liu, H., Kang, S., Jiang, S., & Pang, M. (2023). Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying. Foods, 12(4), 827. https://doi.org/10.3390/foods12040827