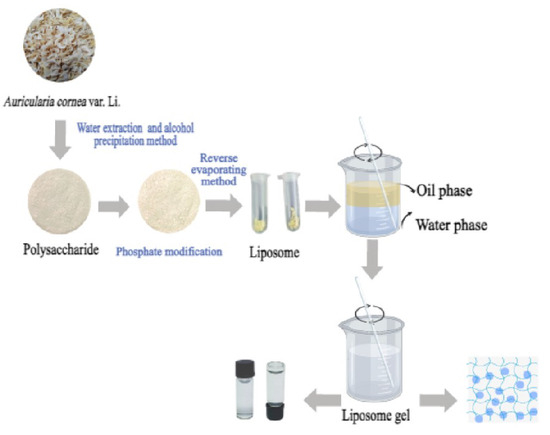
1. Introduction
Edible fungi play a vital role in both nature and the human economy. Polysaccharides are one of the main bioactive components of edible fungi [
1,
2,
3,
4]. A large number of studies have found that edible fungi polysaccharides demonstrate anti-oxidation [
5], immunomodulatory [
6], anti-tumor [
7], hypoglycemic [
8], anti-aging [
9], and other biological activities. ACP is a pure white variant strain with stable genetic traits domesticated and cultivated by the academician Li Yu of Jilin Agricultural University. ACP has good biological effects, including anti-oxidation, anti-inflammation, anti-tumor, liver protection, bacteriostasis, and lowering blood sugar and blood lipids.
The bioactivity of polysaccharides is affected by many factors, and methods are needed to improve their biological activity. Many studies have shown that chemical modification can be used to modify polysaccharides. These modification processes of polysaccharides mainly change the functional groups of polysaccharides by introducing substituents to improve the biological activities of polysaccharides [
10], such as anti-oxidation and anti-aging. The chemical modification methods of polysaccharides mainly include phosphorylation, sulfation, acetylation, alkylation, carboxymethylation, etc. [
11,
12,
13], of which phosphorylation is a common modification method for sugars. Phosphorylation is gentler and simpler than other modification methods. Phosphorylated derivatives are usually prepared by phosphorylating reagents, such as phosphorous acid, phosphoric acid, and polyphosphate, etc. For example, Deng et al. [
14] reported that the preparation of phosphorylated polysaccharides from
Dictyophora indusiata were prepared using a phosphoric acid reagent, and it has been found that phosphorylation can enhance the antioxidant activity of polysaccharides in vitro [
15,
16]. At the same time, in order to effectively improve the bioavailability and biocompatibility of polysaccharides, the material can be prepared at the same time.
Liposomes are a new type of multifunctional self-assembled nanosystem, mainly due to their good biocompatibility, targeting and controllable performance to improve bioavailability and bioactivity, and that they are non-toxic, harmless, and biodegradable, among other advantages [
17,
18,
19]. Due to their unique physiological activity and structural characteristics, liposomes have been widely used as a carrier in medicine, cosmetics, and food [
20]. However, the long storage time of liposomes will lead to particle aggregation, poor stability, and other problems.
The combination of liposomes with biopolymers is a promising method to improve the properties of liposomes in vivo and in vitro. Liposomes are usually encapsulated in a variety of polymer substrates, such as gels, films, and nanofibers. These hybrid systems not only preserve the structural integrity and functionality of the encapsulated liposomes but also offer unique benefits with the synergistic efficiency of two different delivery platforms [
21,
22]. In this study, the gel agent was prepared by dispersing liposomes in the gel matrix. Gels are widely used because they are easy to coat, easy to use, non-toxic, and non-irritating, and they have good biocompatibility and bio-adhesion [
23]. In addition, gels can also improve the stability of polysaccharide liposomes and form multi-reservoir systems to enhance slow release and stability. Attempts have been made to solve the problem of poor stability caused by the long-term storage of liposomes [
24].
This study aimed to solve the problems of the poor solubility, high molecular weight, and biological activity of ACP and to improve the stability of liposomes and maintain their in vitro antioxidant activity. Therefore, liposomes were dispersed in a gel matrix to prepare a liposome gel. Quality evaluation and in vitro antioxidant activity analysis were performed. The authors of this study preliminarily analyzed the liposome gel to further improve the bioavailability of Auricularia cornea var. Li., which can provide a new strategy for the application of Auricularia cornea var. Li.in the deep processing field.
2. Materials and Methods
2.1. Materials
Soybean lecithin and cholesterol were purchased from Shanghai Maclin Biochemical Technology Co., Ltd. (Shanghai, China). Sodium tripolyphosphate, Sodium trimetaphosphate, and glycerol were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Auricularia cornea var. Li. fruit-body was developed and provided by academician Li Yu’s team at Jilin Agricultural University. The other chemicals used were analytically pure.
2.2. Preparation of Phosphorylated Auricularia cornea var. Li. Polysaccharides
2.2.1. Extraction of Auricularia cornea var. Li. Polysaccharides by Water Extract–Alcohol Precipitation
We weighed 3.0 g of Auricularia cornea var. Li. powder and dissolved it in distilled water at a liquid/solid ratio of 70:1 until it fully dissolved. After soaking at room temperature for 12 h, the extraction temperature was set at 70 °C and the extraction time was 3.5 h. The extracted solution was centrifuged at 5000× g for 30 min, and the supernatant was collected. The supernatant was concentrated to 1/4 of the initial volume using a rotary evaporator at 65 °C, and four times the volume of anhydrous ethanol solution was added to the concentrated solution. After alcohol precipitation at 4 °C for 24 h, the ethanol solution was removed by centrifugation (5000× g, 30 min). The obtained polysaccharide was precipitated and fully dissolved with distilled water, concentrated, and the residual ethanol solution was removed. The protein was removed using the Sevag method. After the crude polysaccharide was dissolved, a 1/4 volume of chloroform-n-butanol (4:1, v/v) mixture was added; the mixture was fully shaken for 20 min then left to stand in the separator hopper. The upper water phase was collected, and the extraction was repeated several times until the protein between the water phase and the organic phase was removed. The polysaccharides of Auricularia cornea var. Li. fruit body with the protein removed was obtained by freezing and drying in the upper aqueous phase, which was recorded as ACP.
2.2.2. Preparation of Phosphorylated Auricularia cornea var. Li. Polysaccharides
The phosphorylated reagent (70 mg/mL) was prepared by weighing 5.0 g of sodium tripolyphosphate and 2.0 g of sodium trimetaphosphate in distilled water. We added 1.0 g of ACP and 5.0 g of sodium sulfate, adjusted the pH value to 8.6 after dissolution, and reacted the solution at 80 °C for 5 h. At the end of the reaction, 95% ethanol solution was added to increase the volume of the solution to four times that of the original. Then the solution was precipitated for 24 h, centrifuged at 5000 r/min for 10 min to remove the ethanol, and then redissolved. Then, dialysis (8000 Da) was carried out for 48 h, and the solution was concentrated and lyophilized to obtain phosphorylated Auricularia cornea var. Li. polysaccharide, which was recorded as P-ACP.
2.3. Determination of Phosphate Content
2.3.1. Drawing of Phosphate Standard Curve
The content of phosphate was determined by molybdenum blue colorimetry [
25]. First, 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0 mL of phosphate standard solution (10 μg/mL of potassium dihydrogen phosphate) was added to the corresponding test tube, and ultra-pure water was added to a volume of 5.0 mL. Then, 3.0 mL of phosphoric acid fixing reagent (a phosphoric acid fixing reagent prepared with 1 mL of 20% ascorbic acid, 1 mL of 3 mol/L sulfuric acid, and 1 mL of 3% ammonium molybdate) was immersed in a water bath at 45 °C for 30 min. After cooling, the light absorption value was measured at 660 nm. The phosphoric acid standard curve was drawn with the phosphoric acid concentration as the horizontal coordinate and absorbance as the vertical coordinate. The corresponding curve regression equation was obtained.
2.3.2. Phosphoric Acid Content Determination
The method for determining the content of phosphoric acid in the sample was as follows: We took a 0.5 g sample and placed it in a beaker, added 1.0 mL of concentrated sulfuric acid and 1.0 mL of concentrated nitric acid successively, and heated it until white smoke was produced. After the solution was cooled, we added 1.0 mL of 30% H2O2 and heated it again. We repeated the above operation until no white smoke was generated. Then, we added 1.0 mL of 6 mol/L hydrochloric acid and heated it to decompose the residual acid in the solution. Taking 5.0 mL of P-ACP solution, the content of phosphoric acid in the sample was determined by drawing the standard curve.
Phosphoric acid content = (0.2175 A − 0.0075)/S × 100% formula, where A is the absorbance of the sample measured at 660 nm, and S is the mass (g) of the sample.
2.4. Preparation and Characterization of Liposomes
2.4.1. Preparation of Liposomes
Cholesterol and soy lecithin with a mass ratio of 5:1 were completely dissolved in 20.0 mL of anhydrous ethanol to form an organic phase by the reverse evaporation–ultrasound method. We slowly added an appropriate amount of P-ACP phosphate buffer solution of 2 mg/mL of aqueous phase at pH = 7.4 (we took 1.36 g of dipotassium hydrogen phosphate, added 0.1 mol/L of 79.0 mL of sodium hydroxide solution, and diluted it to 200 mL with water). The mass ratio of soybean lecithin to P-ACP was 15:1. The mixture was stirred continuously and mixed ultrasonically for 15 min, and then the anhydrous ethanol was evaporated under reduced pressure under a water bath at 45 °C. After the mixture formed a colloidal state, PBS was added to 10.0 mL, and the anhydrous ethanol was evaporated under reduced pressure until completely removed, and then ultrasonic homogenization was performed. After passing through a microporous filtration membrane with a pore size of 0.45 um three times and a microporous filtration membrane with a pore size of 0.22 μm once, the polysaccharide liposome suspension was recorded as P-ACPL and refrigerated under a temperature below 4 °C for preservation. Blank liposomes were prepared in the same way (without adding the P-ACP solution).
2.4.2. Determination of Size Distribution, Zeta Potential and Polydispersion Index of Liposomes
We set the mode to zeta or size. Then, we selected the solvent (water) and set the temperature to room temperature and the equilibrium time to 120 s. Then, we selected the sample tank and selected different modes according to the test zeta potential and size. Then, the scanning parameters were set. Parallel scanning was set for each sample three times; the scanning times were 20 s and the delay time was 3 s. The configured sample concentration was 0.5 mg/mL.
2.4.3. Transmission Electron Microscope Observation
An appropriate amount of the sample was dropped on copper mesh (carbon-coated copper mesh) and then the copper mesh was soaked in 0.01 g·mL−1 sodium phosphotungstate solution and stained for 2 min. After removal, the excess solution at the edge was absorbed by filter paper and air-dried. The morphology and structure of the liposomes were observed by transmission electron microscopy.
2.5. Preparation of Phosphorylated Auricularia cornea var. Li. Polysaccharides Liposome Gel
We took an appropriate amount of carbomer 940 and soaked it in water in the refrigerator at 4 °C overnight until it fully swelled. Then, we dropped the triethanolamine with magnetic agitation to adjust the pH value, added an appropriate amount of glycerol to make a gel matrix, an appropriate amount of liposome suspension, an appropriate amount of liposome suspension, and an appropriate amount of water to the gel matrix, and then evenly ground the liposome gel to make P-ACPLG.
2.6. Uniform Experimental Design
2.6.1. Design of Comprehensive Evaluation Form
The 2015 edition of the Chinese Pharmacopoeia (Part 4) stipulates that the appearance of the gel should be uniform, delicate, that it can remain gelatinous at room temperature, and that it will not dry out or liquefy. Therefore, the evaluation indexes were determined as the appearance of the gel, its coating ductility, uniformity, discoloration time, and viscosity. The specific evaluation indexes are shown in
Table 1, and the molding of the gel was studied. Prior to the test session, the grading team members were given a brief description of the overall grading procedure. The coded gels were provided to the ethics committee of Jilin Agricultural University (232692HJ0102116492, 10 May 2023) for the trained sensory evaluation members (
n = 10) in random order in centrifuge tubes.
2.6.2. Determination of Entrapment Efficiency
The centrifugal method was used to determine the entrapment efficiency. First, 1.0 mL of polysaccharide liposome gel was precisely measured in a centrifuge tube, placed in a frozen centrifuge, and centrifuged at 10,000 r·min
−1 for 30 min. After absorbing 0.5 mL of supernatant and adding PBS solution to 100.0 mL, the total polysaccharide content was determined by the phenol-concentrated sulfuric acid method [
26].
2.6.3. Uniform Design Experiment
Factors such as the dosage of carbomer 940, liposome, glycerol, and pH value were investigated, and the encapsulation rate and comprehensive score were used as indicators to select six levels (pseudo-level) for each factor. Other factors affecting the quality of the liposomes were tested under fixed conditions and parallel operation methods according to uniform design table U6(6
4), as shown in
Table 2.
2.7. Storage Stability
To determine the effect of storage conditions on the leakage rate of the liposome and liposome gel, we transferred 4 mL from each sample into test tubes and stored them at refrigerated temperature (−4 °C), room temperature (25 °C), and frozen temperature (−20 °C) for 28 days. The frozen sample was melted by repeated eddy mixing at room temperature. Then, the change in the sample leakage rate was measured.
2.8. In Vitro Drug Release Experiment
In vitro drug release was measured by dialysis. Neutral PBS (pH = 7.4) was selected as the release medium, and 2 mL 1 mg/mL of phosphorylated Auricularia cornea var. Li. polysaccharides and phosphorylated Auricularia cornea var. Li. polysaccharide liposomes were added into the dialysis bag (3000 KD), respectively. Both ends of the dialysis bag were sealed and placed in a 20 mL release medium at 37 °C ± 0.5 °C to simulate the skin environment. At specific intervals (1, 2, 4, 6, 8, 10, 12, 24, 48, 72, 96 h), 2 mL of the release medium was removed each time into a 10 mL centrifuge tube, and an equal amount of new release medium was added to the beaker. The sample in the centrifuge tube was quantitatively determined by the phenol-concentrated sulfuric acid method (n = 3).
2.9. Determination of Antioxidant Activity In Vitro
2.9.1. Determination of DPPH Free Radical Scavenging Ability
The samples (ACP, P-ACP, P-ACPL, P-ACPLG) were prepared (0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 2.0 mg/mL, 4.0 mg/mL) at 1 mL with different concentration gradients. We added 1.0 mL of 0.2 mmol/L of DPPH radical ethanol solution (ready to use) and 1.0 mL of the sample solution, shook it well, and placed the prepared solution in room temperature for 30 min away from light. With anhydrous ethanol as the blank control and L-ascorbic acid as the positive control, the absorbance value was measured at 517 nm and the DPPH free radical clearance was calculated. The calculation formula is as follows:
where
X represents the DPPH free radical clearance, %;
A1 represents the absorbance value of the blank control (anhydrous ethanol replaces the sample);
A2 represents the absorbance value of the sample test; and
A0 represents the absorbance value of the sample interference test (anhydrous ethanol instead of the DPPH solution).
2.9.2. Determination of Scavenging Ability of Hydroxyl Radical (·OH)
We added 1 mL samples (ACP, P-ACP, P-ACPL, P-ACPLG) of different concentrations (0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 2.0 mg/mL, 4.0 mg/mL) and ferrous sulfate (1.0 mL, 6 mmol/L) into test tubes successively. Salicylic acid (1.0 mL, 6 mmol/L) and 1 mL of hydrogen peroxide (0.03%,
v:
v) were shook and mixed. Then, they were placed in a 37 °C water bath for 30 min with anhydrous ethanol as a blank control and L-ascorbic acid as a positive control. The absorbance value was determined at 510 nm.
where
X represents the hydroxyl radical clearance, %;
A1 represents the absorbance value of the blank control (anhydrous ethanol replaces the sample);
A2 represents the absorbance value of the sample test; and
A0 indicates the absorbance value of the sample interference test (anhydrous ethanol instead of hydrogen peroxide solution).
2.9.3. Reducing Power Test
The 1.0 mL sample (ACP, P-ACP, P-ACPL, P-ACPLG) solution and 1.0 mL potassium ferricyanide solution (1%, w/v) were added to the test tube successively. After full shock and mixing, the reaction was carried out in a water bath at 50 °C for 30 min. After rapid cooling with running water, 1.0 mL of 10% trichloroacetic acid was added. After centrifugation at 6000 r/min for 10 min, 500 μL of supernatant was added to 30 μL of ferric chloride solution (0.1%, w/v), mixed, and reacted at room temperature for 5 min. Anhydrous ethanol was used as the blank control and L-ascorbic acid was used as the positive control. The absorbance value was determined at 700 nm.
2.10. Data Processing
All experiments were measured and repeated three times. Origin 2018 software was used for mapping and DPS7.5 software was used for stepwise regression analysis of experimental data. Significant differences were considered when p < 0.05.
4. Conclusions
The preparation and in vitro antioxidant activity of P-ACPLG were studied in this paper. The results show that P-ACPL prepared by the reverse evaporation method is spherical in appearance. The experimental results of the storage stability of P-ACPL show that the storage stability of P-ACPL at 4 °C is higher than that at room temperature and -20 °C. However, due to the accumulation of colloidal particles caused by the long-term storage of liposomes, the P-ACPLG was prepared by dispersing liposomes into a gel matrix. The experimental results of the storage stability of P-ACPLG show that it is suitable for storage at 4 °C, and has the characteristics of a low leakage rate and strong stability. The results of its in vitro antioxidant activity show that with the increase in the P-ACPL concentration, the scavenging rate of DPPH free radicals and hydroxyl free radicals and the reducing ability of P-ACPL increased gradually, and the scavenging rate of P-ACPL was slightly stronger than that of ACP and P-ACP. After emulsifying the P-ACPL into P-ACPLG and coating the gel matrix, the DPPH, hydroxyl radical scavenging rate, and reducing ability of the P-ACPL were unchanged. In future studies, P-ACPLG can be used as a new type of antioxidant. The mechanism of liposome gel on the skin needs further study.