Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System
Abstract
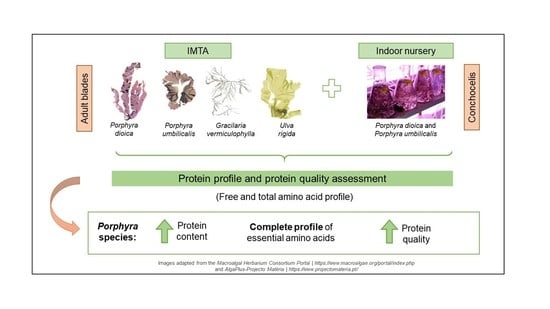
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Algal Biomass
2.3. Amino Acid Composition Analysis
2.3.1. Total Amino Acids Extraction
2.3.2. Free Amino Acids Extraction
2.3.3. Chromatographic Analysis
2.4. Evaluation of Protein Quality
2.5. Determination of Total Nitrogen, Protein Nitrogen, and Non-protein Nitrogen
2.6. Statistical Analysis
3. Results and Discussion
3.1. Amino Acids Composition
3.1.1. Total Amino Acids
3.1.2. Essential Amino Acids
3.1.3. Non-Essential Amino Acids
3.1.4. Free Amino Acids
3.2. Evaluation of Protein Quality Based on the Amino Acids Profile
3.3. Nitrogen and Protein Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Satish, L.; Rameshkumar, R.; Rathinapriya, P.; Pandian, S.; Rency, A.S.; Sunitha, T.; Ramesh, M. Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J. Appl. Phycol. 2015, 27, 993–1002. [Google Scholar] [CrossRef]
- Satish, L.; Santhakumari, S.; Gowrishankar, S.; Pandian, S.K.; Ravi, A.V.; Ramesh, M. Rapid biosynthesized AgNPs from Gelidiella acerosa aqueous extract mitigates quorum sensing mediated biofilm formation of Vibrio species—An in vitro and in vivo approach. Environ. Sci. Pollut. Res 2017, 24, 27254–27268. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. Environ. Biol. Fishes 2016, 29, 949–982. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Kazir, M.; AbuHassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.; Oliva-Teles, M.T.; Carvalho, A.P.; Domingues, V.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino Acids: Biochemistry and Nutrition, 1st ed.; Wu, G., Ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Singhal, N.K.; Freeman, E.; Arning, E.; Wasek, B.; Clements, R.; Sheppard, C.; Blake, P.; Bottiglieri, T.; McDonough, J. Dysregulation of methionine metabolism in multiple sclerosis. Neurochem. Int. 2018, 112, 1–4. [Google Scholar] [CrossRef]
- Chen, S.-F.; Pan, M.-X.; Tang, J.-C.; Cheng, J.; Zhao, D.; Zhang, Y.; Liao, H.-B.; Liu, R.; Zhuang, Y.; Zhang, Z.-F.; et al. Arginine is neuroprotective through suppressing HIF-1α/LDHA-mediated inflammatory response after cerebral ischemia/reperfusion injury. Mol. Brain 2020, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-Y.; Kim, E.-H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. In Globefish Research Programme; Food and Agriculture Organization of the United Nations: Quebec City, QC, Canada, 2018; Volume 124, p. I. [Google Scholar]
- Stévant, P.; Rebours, C.; Chapman, A. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rosa, J.; Lemos, M.F.; Crespo, D.; Nunes, M.; Freitas, A.; Ramos, F.; Pardal, M.Â.; Leston, S. Integrated multitrophic aquaculture systems—Potential risks for food safety. Trends Food Sci. Technol. 2020, 96, 79–90. [Google Scholar] [CrossRef]
- Kleitou, P.; Kletou, D.; David, J. Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 2018, 490, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; De Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. Environ. Biol. Fishes 2015, 28, 511–524. [Google Scholar] [CrossRef]
- Silva, D.M.; Valente, L.M.; Pinto, I.S.; Pereira, R.; Pires, M.; Seixas, F.; Rema, P. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. Environ. Biol. Fishes 2014, 27, 1671–1680. [Google Scholar] [CrossRef]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef]
- Chen, J.; Pu, X. Cultured Aquatic Species Information Programme. Porphyra spp.; Cultured Aquatic Species Information Programme in FAO Fisheries Division; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Available online: http://www.fao.org/fishery/culturedspecies/Porphyra_spp/en (accessed on 11 February 2020).
- Abreu, H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Pinto, I.S. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Lopes, D.; Moreira, A.S.; Rey, F.; Da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. Environ. Biol. Fishes 2018, 31, 1369–1381. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.; Costa, A.S.G.; Pimentel, F.B.; Oliveira, M.B.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef] [PubMed]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Protein and Amino Acid Nutrition; Elsevier: Amsterdam, The Netherlands, 1959; pp. 281–295. [Google Scholar]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint FAO/WHO/UNU Expert Consultation; Technical Report Series 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- AOAC. Official Method of Analysis: Association of Analytical Chemists, 19th ed.; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.-J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. Environ. Biol. Fishes 2016, 29, 1001–1009. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. Environ. Biol. Fishes 2012, 25, 677–685. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical composition and physicochemical properties of tropical red seaweed. Gracilaria Changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Filho, J.M.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Astorga-España, M.S.; Rodríguez-Galdón, B.; Rodríguez, E.M.R.; Díaz-Romero, C. Amino acid content in seaweeds from the Magellan Straits (Chile). J. Food Compos. Anal. 2016, 53, 77–84. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef]
- Lin, R.; Stekoll, M.S. Phycobilin content of the conchocelis phase of alaskan Porphyra (Bangiales, Rhodophyta) species: Responses to environmental variables. J. Phycol. 2010, 47, 208–214. [Google Scholar] [CrossRef]
- Mukai, L.S.; Craigie, J.S.; Brown, R.G. Chemical composition and structure of the cell walls of the conchocelis and thallus phases of Porphyra tenera (Rhodophyceae). J. Phycol. 2008, 17, 192–198. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef]
- Friedman, M. Nutritional value of proteins from different food sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- López-Lopez, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larreamarin, M.; Sánchez-Muniz, F.; Cofrades, S.; Colmenero, F.J. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Gaillard, C.; Bhatti, H.S.; Novoa-Garrido, M.; Lind, V.; Roleda, M.Y.; Weisbjerg, M. Amino acid profiles of nine seaweed species and their In Situ degradability in dairy cows. Anim. Feed. Sci. Technol. 2018, 241, 210–222. [Google Scholar] [CrossRef]
- Mišurcová, L.; Buňka, F.; Ambrožová, J.V.; Machů, L.; Samek, D.; Kračmar, S. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014, 151, 120–125. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and functional bioactivity value of selected azorean macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.D.S.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional value of commercial protein-rich plant products. Plant Foods Hum. Nutr. 2018, 73, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2008, 37, 105–110. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.-J.; Frosch, M.P.; Von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef] [Green Version]
- Angell, A.R.; Mata, L.; De Nys, R.; Paul, N.A. Variation in amino acid content and its relationship to nitrogen content and growth rate in Ulva ohnoi (Chlorophyta). J. Phycol. 2014, 50, 216–226. [Google Scholar] [CrossRef]
- Noda, H.; Horiguchi, Y.; Araki, S. Studies on the flavor substances of “nori”, the dried laver Porphyra spp. II. Free amino acids and 5”-nucleotides. Bull. Jpn. Soc. Sci. Fish. 1975, 41, 1299–1303. [Google Scholar] [CrossRef]
- Admassu, H.; Abera, T.; Abraha, B.; Yang, R.; Zhao, W. Proximate, mineral and amino acid composition of dried laver (Porphyra spp.) seaweed. J. Acad. Ind. Res. 2018, 6, 149. [Google Scholar]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Benjama, O.; Payap, M. Biochemical composition and physicochemical properties of two red seaweeds (Gracilaria fisheri and G. tenuistipitata) from the Pattani Bay in Southern Thailand. Songklanakarin J. Sci. Technol. 2012, 34, 223. [Google Scholar]
- Brown, M.R.; Jeffrey, S. Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 1. Amino acids, sugars and pigments. J. Exp. Mar. Biol. Ecol. 1992, 161, 91–113. [Google Scholar] [CrossRef]
- World Health Organization. Iodine Deficiency in Europe: A Continuing Public Health Problem; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
| Amino Acids | Porphyra dioica | Porphyra umbilicalis | Gracilaria vermiculophylla (mg/g ds) | Ulva rigida (mg/g ds) | ||
|---|---|---|---|---|---|---|
| Blades (mg/g ds) | Conchocelis (mg/g ds) | Blades (mg/g ds) | Conchocelis (mg/g ds) | |||
| Asp | 24.15 ± 0.64 bc | 32.49 ± 0.21 a | 23.69 ± 0.65 c | 26.61 ± 1.75 b | 12.68 ± 0.41 d | 12.05 ± 0.29 d |
| Glu | 22.57 ± 0.72 c | 31.48 ± 0.43 a | 21.07 ± 0.22 c | 25.91 ± 1.63 b | 12.47 ± 0.54 d | 9.47 ± 0.23 e |
| Ala | 23.30 ± 0.79 bc | 30.25 ± 0.29 a | 21.76 ± 0.17 b | 23.52 ± 1.45 b | 8.11 ± 0.35 c | 8.48 ± 0.10 c |
| Arg | 14.58 ± 0.54 c | 23.68 ± 0.24 a | 14.27 ± 0.11 c | 17.88 ± 1.23 b | 8.37 ± 0.35 d | 6.06 ± 0.07 e |
| Gly | 16.75 ± 0.74 a | 18.22 ± 0.22 a | 13.61 ± 0.09 b | 16.65 ± 0.93 a | 6.82 ± 0.36 c | 6.67 ± 0.65 c |
| Ser | 12.05 ± 0.44 bc | 16.42 ± 0.20 a | 10.54 ± 0.12 c | 13.26 ± 0.80 b | 6.75 ± 0.22 d | 5.54 ± 0.06 d |
| Tyr | 6.15 ± 0.25c | 10.17 ± 0.16 a | 5.47 ± 0.09 c | 8.75 ± 0.59 b | 3.53 ± 0.13 d | 3.25 ± 0.07 d |
| Pro | 9.08 ± 0.32 a | 9.79 ± 0.32 a | 8.60 ± 0.08 a | 8.73 ± 0.71 a | 4.82 ± 0.18 b | 4.40 ± 0.10 b |
| Hyp | 0.15 ± 0.01 c | 0.05 ± 0.01 d | n.d. | 0.05 ± <0.01 d | 0.26 ± 0.01 b | 1.06 ± 0.02 a |
| Phe | 9.27 ± 0.35 b | 11.68 ± 0.07 a | 8.56 ± 0.07 b | 9.12 ± 0.58 b | 6.15 ± 0.30 c | 5.79 ± 0.03 c |
| His | 2.05 ± 0.50 c | 6.62 ± 0.08 a | 1.66 ± 0.05 cd | 6.46 ± 0.29 a | 1.14 ± 0.04 d | 2.96 ± 0.07 b |
| Ile | 8.22 ± 0.34 b | 11.29 ± 0.17 a | 8.31 ± 0.07 b | 8.42 ± 0.53 b | 5.86 ± 0.28 c | 4.44 ± 0.06 d |
| Leu | 16.63 ± 0.62 b | 22.03 ± 0.25 a | 16.12 ± 0.15 b | 17.32 ± 1.03 b | 9.01 ± 0.39 c | 7.89 ± 0.09 c |
| Lys | 11.60 ± 1.15 c | 22.33 ± 0.35 a | 11.58 ± 0.19 c | 16.08 ± 0.68 b | 5.80 ± 0.19 d | 4.76 ± 0.70 d |
| Met | 2.22 ± 0.24 c | 5.49 ± 0.05 a | 2.40 ± 0.04 c | 4.73 ± 0.28 b | 1.37 ± 0.07 d | 1.92 ± 0.05 c |
| Thr | 12.27 ± 0.34 b | 14.82 ± 0.15 a | 12.13 ± 0.09 b | 11.87 ± 0.66 b | 6.21 ± 0.24 c | 4.88 ± 0.14 d |
| Trp | 0.59 ± 0.03 d | 1.50 ± 0.05 a | 0.79 ± 0.03 c | 1.37 ± 0.02 b | 0.42 ± 0.01 e | 0.85 ± 0.02 c |
| Val | 12.34 ± 0.42 b | 18.24 ± 0.25 a | 12.79 ± 0.09 b | 13.63 ± 0.84 b | 6.84 ± 0.35 c | 5.78 ± 0.03 c |
| ∑TAA | 203.99 ± 8.20 c | 286.56 ± 3.11 a | 193.34 ± 2.16 c | 230.34 ± 13.79 b | 106.62 ± 4.09 d | 96.22 ± 0.78 d |
| % EAA | 36.84 ± 0.44 d | 39.78 ± 0.06 b | 38.45 ± 0.10 c | 38.65 ± 0.28 c | 40.14 ± 0.09 ab | 40.79 ± 0.23 a |
| % NEAA | 63.16 ± 0.44 a | 60.22 ± 0.06 c | 61.55 ± 0.10 b | 61.35 ± 0.28 b | 59.86 ± 0.09 cd | 59.21 ± 0.23 d |
| EAA/NEAA | 0.58 ± 0.01 d | 0.66 ± <0.01 b | 0.62 ± <0.01 c | 0.63 ± 0.01 c | 0.67 ± <0.01 ab | 0.69 ± 0.01 a |
| Amino Acids | Porphyra dioica | Porphyra umbilicalis | Gracilaria vermiculophylla (mg/g ds) | Ulva rigida (mg/g ds) | ||
|---|---|---|---|---|---|---|
| Blades (mg/g ds) | Conchocelis (mg/g ds) | Blades (mg/g ds) | Conchocelis (mg/g ds) | |||
| Asp | 2.23 ± 0.01 b | 1.30 ± 0.01 c | 3.53 ± 0.09 a | 1.44 ± 0.09 c | 0.53 ± 0.03 d | 0.24 ± <0.01 e |
| Glu | 3.67 ± 0.05 b | 4.63 ± 0.03 a | 3.15 ± 0.08 c | 4.77 ± 0.15 a | 1.14 ± 0.03 d | 0.21 ± 0.01 e |
| Asn | 0.55 ± 0.01 b | 0.35 ± <0.01 d | 0.44 ±0.01 c | 0.32 ± 0.02 d | 0.14 ± <0.01 e | 1.45 ± 0.06 a |
| Gln | 0.09 ± <0.01 d | 0.33 ± <0.01 a | 0.14 ± <0.01 b | 0.13 ± 0.01 c | 0.08 ± <0.01 e | 0.08 ± <0.01 de |
| Ala | 5.50 ± 0.06 a | 5.37 ± 0.04 a | 5.01 ± 0.08 b | 3.70 ± 0.13 c | 0.26 ± 0.01 d | 0.19 ± 0.01 d |
| Arg | 0.12 ± 0.01 c | 0.70 ± 0.01 a | 0.14 ± <0.01 c | 0.29 ± 0.01 b | 0.13 ± 0.02 c | 0.08 ± <0.01 d |
| Gly | 0.13 ± 0.01 d | 0.37 ± <0.01 a | 0.15 ± 0.01 cd | 0.29 ± 0.05 b | 0.10 ± 0.01 d | 0.20 ± 0.01 c |
| Ser | 0.26 ± <0.01 c | 0.45 ± 0.01 b | 0.29 ± <0.01 c | 0.77 ± 0.03 a | 0.19 ± 0.01 d | 0.13 ± <0.01 e |
| Tyr | 0.04 ± <0.01 c | 0.18 ± <0.01 a | 0.07 ± 0.01 b | 0.05 ± <0.01 c | 0.07 ± <0.01 b | 0.07 ± <0.01 b |
| Pro | 0.04 ± <0.01 de | 0.14 ± 0.01 a | 0.03 ± <0.01 e | 0.12 ± 0.01 b | 0.05 ± 0.01 d | 0.07 ± <0.01 c |
| Hyp | 0.01 ± <0.01 b | n.d. | 0.01 ± <0.01 b | n.d. | 0.01 ± <0.01 a | 0.01 ± <0.01 a |
| Phe | 0.05 ± <0.01 d | 0.27 ± <0.01 a | 0.08 ± <0.01 c | 0.11 ± <0.01 b | 0.05 ± <0.01 d | 0.04 ± <0.01 d |
| His | 0.18 ± 0.01 b | 0.06 ± <0.01 c | 0.02 ± <0.01 c | 0.03 ± 0.01 c | 0.20 ± 0.02 b | 1.96 ± 0.09 a |
| Ile | 0.07 ± <0.01 b | 0.17 ± <0.01 a | 0.07 ± <0.01 b | 0.08 ± <0.01 b | 0.05 ± <0.01 c | 0.04 ± <0.01 c |
| Leu | 0.07 ± <0.01 c | 0.39 ± <0.01 a | 0.07 ± <0.01 c | 0.13 ± 0.01 b | 0.04 ± <0.01 d | 0.05 ± <0.01 d |
| Lys | 0.11 ± <0.01 cd | 0.37 ± 0.01 a | 0.13 ± <0.01 c | 0.25 ± 0.03 b | 0.10 ± 0.01 cd | 0.07 ± <0.01 d |
| Met | n.d. | 0.16 ± <0.01 | n.d. | n.d. | n.d. | n.d. |
| Thr | 0.28 ± <0.01 d | 0.46 ± 0.01 b | 0.41 ± 0.01 c | 0.53 ± 0.03 a | 0.16 ± 0.01 e | n.d. |
| Trp | n.d. | 0.09 ± <0.01 | n.d. | n.d. | n.d. | n.d. |
| Val | 0.16 ± 0.01 c | 0.37 ± <0.01 a | 0.14 ± <0.01 c | 0.21 ± 0.01 b | 0.08 ± 0.01 d | 0.06 ± <0.01 e |
| ∑FAA | 13.57 ± 0.15 bc | 16.17 ± 0.03 a | 13.88 ± 0.28 b | 13.20 ± 0.23 c | 3.36 ± 0.11 e | 4.95 ± 0.15 d |
| Essential Amino Acids | Amino Acids Scoring Pattern | Porphyra dioica | Porphyra umbilicalis | Gracilaria vermiculophylla | Ulva rigida | ||
|---|---|---|---|---|---|---|---|
| (mg AA/g Protein) [27] | Blades | Conchocelis | Blades | Conchocelis | |||
| His | 15 | 9.96 ± 2.61 c | 23.09 ± 0.11 b | 8.56 ± 0.19 c | 28.05 ± 0.67 a | 10.70 ± 0.29 c | 30.71 ± 0.64 a |
| Ile | 30 | 40.30 ± 0.07 d | 39.39 ± 0.22 d | 42.99 ± 0.22 c | 36.55 ± 0.15 e | 54.94 ± 0.66 a | 46.11 ± 0.87 b |
| Leu | 59 | 81.52 ± 0.30 b | 76.86 ± 0.05 cd | 83.40 ± 0.22 ab | 75.21 ± 0.32 c | 84.53 ± 0.52 a | 82.02 ± 1.71 b |
| Lys | 45 | 56.76 ± 4.36 c | 77.94 ± 0.81 a | 59.88 ± 0.58 bc | 69.92 ± 2.93 ab | 54.40 ± 0.36 c | 49.37 ± 8.47 c |
| Met | 22 * | 10.87 ± 1.05 b | 19.17 ± 0.09 a | 12.43 ± 0.22 b | 20.54 ± 0.21 a | 12.88 ± 1.33 b | 19.93 ± 0.77 a |
| Phe + Tyr | 38 | 75.61 ± 0.14 d | 76.24 ± 0.23 cd | 72.60 ± 0.27 e | 77.53 ± 0.59 c | 90.81 ± 0.57 b | 93.90 ± 1.09 a |
| Thr | 23 | 60.17 ± 0.96 ab | 51.71 ± 0.10 cd | 62.73 ± 0.42 a | 51.54 ± 0.53 c | 58.24 ± 0.23 b | 50.78 ± 2.21 c |
| Trp | 6 | 2.92 ± 0.25 d | 5.23 ± 0.27 b | 4.09 ± 0.26 c | 5.96 ± 0.35 b | 3.97 ± 0.17 c | 8.80 ± 0.37 a |
| Val | 39 | 60.50 ± 0.47 c | 63.65 ± 0.39 b | 66.14 ± 0.32 a | 59.16 ± 0.65 c | 64.09 ± 1.01 b | 60.06 ± 0.85 c |
| LAA | - | Trp | Met | Met | Met | Met | Met |
| AAS (%) | - | 48.7 | 87.1 | 56.5 | 93.4 | 58.4 | 90.8 |
| EAAI (%) | - | 90.8 | 114.2 | 96.5 | 115.7 | 101.9 | 123.4 |
| Nitrogen/Protein | Porphyra dioica | Porphyra umbilicalis | Gracilaria vermiculophylla (% ds) | Ulva rigida (% ds) | ||
|---|---|---|---|---|---|---|
| Blades (% ds) | Conchocelis (% ds) | Blades (% ds) | Conchocelis (% ds) | |||
| NPN | 1.12 ± 0.04 a | 0.88 ± 0.01 ab | 0.64 ± 0.01 ab | 0.46 ± 0.14 ab | 0.44 ± 0.23 ab | 0.22 ± 0.17 b |
| PN | 3.62 ± 0.01 c | 4.47 ± <0.01 a | 3.99 ± 0.01 bc | 4.33 ± 0.14 ab | 2.24 ± <0.01 d | 1.82 ± 0.14 d |
| TN | 4.74 ± 0.05 b | 5.35 ± 0.01 a | 4.62 ± <0.01 b | 4.79 ± <0.01 b | 2.68 ± 0.23 c | 2.04 ± 0.03 d |
| Crude protein | 23.70 ± 0.26 b | 26.73 ± 0.03 a | 23.11 ± <0.01 b | 23.97 ± 0.01 b | 13.38 ± 1.16 c | 10.19 ± 0.16 d |
| True protein (NP × 5) | 18.10 ± 0.05 c | 22.34 ± 0.02 a | 19.93 ± 0.03 bc | 21.67 ± 0.71 ab | 11.19 ± <0.01 d | 9.08 ± 0.71 d |
| True protein (∑AAT) | 20.40 ± 0.82 c | 28.66 ± 0.31 a | 19.33 ± 0.22 c | 23.03 ± 1.38 b | 10.66 ± 0.41 d | 9.62 ± 0.08 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382. https://doi.org/10.3390/foods9101382
Machado M, Machado S, Pimentel FB, Freitas V, Alves RC, Oliveira MBPP. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods. 2020; 9(10):1382. https://doi.org/10.3390/foods9101382
Chicago/Turabian StyleMachado, Marlene, Susana Machado, Filipa B. Pimentel, Victor Freitas, Rita C. Alves, and M. Beatriz P. P. Oliveira. 2020. "Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System" Foods 9, no. 10: 1382. https://doi.org/10.3390/foods9101382
APA StyleMachado, M., Machado, S., Pimentel, F. B., Freitas, V., Alves, R. C., & Oliveira, M. B. P. P. (2020). Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods, 9(10), 1382. https://doi.org/10.3390/foods9101382