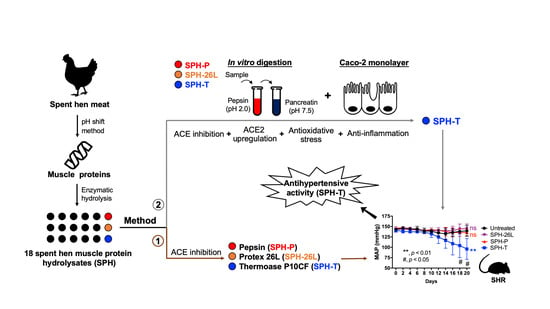
Spent Hen Protein Hydrolysate with Good Gastrointestinal Stability and Permeability in Caco-2 Cells Shows Antihypertensive Activity in SHR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPHs
2.3. Analysis of Protein Content, Hydrolysis Yield, and Degree of Hydrolysis (DH) of SPHs
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Size Exclusion Chromatography
2.6. Desalting Protocol, Simulated Gastrointestinal Digestion, and ACE Inhibitory Assay of SPHs
2.7. Cell Culture of A7r5, EA.hy926, and Caco-2 Cells
2.8. Western Blotting
2.9. Superoxide Detection
2.10. Caco-2 Transport of the Gastrointestinal-Digested SPH
2.11. Identification of Peptides of Caco-2 Permeates
2.12. Cytotoxicity
2.13. Ethics Statement, Animal Model and Telemetry Recording
2.14. Statistical Analysis
3. Results
3.1. Hydrolysis Yield, Protein Content, DH, and ACE Inhibition of SPHs
3.2. Molecular Weight Distribution of SPHs
3.3. ACE2 Upregulating, Antioxidant, and Anti-Inflammatory Activity of SPH-P, SPH-26L, and SPH-T
3.4. Effect of Gastrointestinal Digestion on ACE Inhibitory, ACE2 Upregulating, Antioxidant, and Anti-Inflammatory Activity of SPH-P, SPH-26L, and SPH-T
3.5. Effect of Caco-2 Transport on ACE Inhibitory, ACE2 Upregulating, Antioxidant, and Anti-Inflammatory Activities of SPH-TPP (pepsin-pancreatin digested spent hen hydrolysate prepared by thermoase)
3.6. Antihypertensive Effect of SPHs in SHRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanna, A.; Lefkowitz, L.; White, W.B. Evaluation of recent fixed-dose combination therapies in the management of hypertension. Curr. Opin. Nephrol. Hypertens. 2008, 17, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ritchey, M.D.; Gillespie, C.; Wozniak, G.; Shay, C.M.; Thompson-Paul, A.M.; Loustalot, F.; Hong, Y. Potential need for expanded pharmacologic treatment and lifestyle modification services under the 2017 ACC/AHA Hypertension Guideline. J. Clin. Hypertens. 2018, 20, 1377–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Xi, B.; Zhao, M.; Wang, L.; Veeranki, S.P. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: The NHANES III Linked Mortality Study. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yokoyama, K.; Yoshikawa, M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar]
- Jensen, I.J.; Eysturskarð, J.; Madetoja, M.; Eilertsen, K.E. The potential of cod hydrolyzate to inhibit blood pressure in spontaneously hypertensive rats. Nutr. Res. 2014, 34, 168–173. [Google Scholar] [CrossRef]
- Fan, H.; Xu, Q.; Hong, H.; Wu, J. Stability and transport of spent hen-derived ACE-inhibitory peptides IWHHT, IWH, and IW in human intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2018, 66, 11347–11354. [Google Scholar] [CrossRef]
- Miguel, M.; Dávalos, A.; Manso, M.A.; de la Peña, G.; Lasunción, M.A.; López-Fandiño, R. Transepithelial transport across Caco-2 cell monolayers of antihypertensive egg-derived peptides PepT1-mediated flux of Tyr-Pro-Ile. Mol. Nutr. Food Res. 2008, 52, 1507–1513. [Google Scholar] [CrossRef]
- Liang, Q.; Chalamaiah, M.; Ren, X.; Ma, H.; Wu, J. Identification of new anti-inflammatory peptides from zein hydrolysate after simulated gastrointestinal digestion and transport in Caco-2 cells. J. Agric. Food Chem. 2018, 66, 1114–1120. [Google Scholar] [CrossRef]
- Ewart, H.S.; Dennis, D.; Potvin, M.; Tiller, C.; Fang, L.-H.; Zhang, R.; Zhu, X.-M.; Curtis, J.M.; Cloutier, S.; Du, G. Development of a salmon protein hydrolysate that lowers blood pressure. Eur. Food Res. Technol. 2009, 229, 561–569. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. A new approach for identification of novel antihypertensive peptides from egg proteins by QSAR and bioinformatics. Food Res. Int. 2010, 43, 1371–1378. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, Y.; Bai, J.; Wu, W.; Lin, Q.; Wu, J. Spent hen-derived ACE inhibitory peptide IWHHT shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2019, 53, 85–92. [Google Scholar] [CrossRef]
- Tanaka, M.; Hong, S.M.; Akiyama, S.; Hu, Q.Q.; Matsui, T. Visualized absorption of anti-atherosclerotic dipeptide, Trp-His, in Sprague–Dawley rats by LC-MS and MALDI-MS imaging analyses. Mol. Nutr. Food Res. 2015, 59, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; Lopez-Fandino, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and anti hypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef]
- Miguel, M.; Manso, M.A.; Martin-Alvarez, P.J.; Aleixandre, A.; Lopez-Fandino, R. Angiotensin-converting enzyme activity in plasma and tissues of spontaneously hypertensive rats after the short- and long-term intake of hydrolysed egg white. Mol. Nutr. Food Res. 2007, 51, 555–563. [Google Scholar] [CrossRef]
- Pan, H.; She, X.; Wu, H.; Ma, J.; Ren, D.; Lu, J. Long-term regulation of the local renin–angiotensin system in the myocardium of spontaneously hypertensive rats by feeding bioactive peptides derived from Spirulina platensis. J. Agric. Food Chem. 2015, 63, 7765–7774. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Tech. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Davidge, S.T.; Wu, J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/Ang (1–7)/Mas receptor axis. Mol. Nutr. Food Res. 2019, 63, 1900063. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Majumder, K.; Li, Q.Y.; Panahi, S.; Morton, J.S.; Davidge, S.T.; Wu, J.P. Egg white protein hydrolysate reduces blood pressure, improves vascular relaxation and modifies aortic angiotensin II receptors expression in spontaneously hypertensive rats. J. Funct. Foods 2016, 27, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Tsai, B.C.-K.; Hsieh, D.J.-Y.; Lin, W.-T.; Tamilselvi, S.; Day, C.H.; Ho, T.-J.; Chang, R.-L.; Viswanadha, V.P.; Kuo, C.-H.; Huang, C.-Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.-J.; Wang, Z.; Xing, C.-R.; Yuan, J.; Wang, L.-F.; Udenigwe, C.; Ju, X.-R. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. NPJ Sci. Food 2019, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbandeh, M. Egg Production Worldwide 1990–2018. Available online: https://www.statista.com/statistics/263972/egg-production-worldwide-since-1990/ (accessed on 1 September 2020).
- Wu, J. Eggs and egg products processing. Food Process. Princ. Appl. 2014, 437–455. [Google Scholar]
- Liao, W.; Jahandideh, F.; Fan, H.; Son, M.; Wu, J. Egg Protein-Derived Bioactive Peptides: Preparation, Efficacy, and Absorption. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–58. [Google Scholar]
- Ahmed, T.A.; Kulshreshtha, G.; Hincke, M.T. Value-Added Uses of Eggshell and Eggshell Membranes. In Eggs as Functional Foods and Nutraceuticals for Human Health; Royal Society of Chemistry: London, UK, 2019; pp. 359–397. [Google Scholar]
- AAFC (Agriculture and Agri-Food Canada). Poult Egg Mark. Inf.: Rep. 108–Chicks/Poults Destroyed. Available online: https://aimis-simia.agr.gc.ca/rp/index-eng.cfm?action=pR&r=207&pdctc= (accessed on 30 September 2019).
- Shahbandeh, M. Total Number of Laying Hens in the US 2000–2019. Available online: https://www.statista.com/statistics/195823/total-number-of-laying-hens-in-the-us-since-2000/ (accessed on 1 September 2020).
- Wang, H.; Wu, J.; Betti, M. Chemical, rheological and surface morphologic characterisation of spent hen proteins extracted by pH-shift processing with or without the presence of cryoprotectants. Food Chem. 2013, 139, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J. Preparation and characterization of adhesive from spent hen proteins. Int. J. Adhes. Adhes. 2012, 36, 8–14. [Google Scholar] [CrossRef]
- Zubair, M.; Wu, J.; Ullah, A. Hybrid bionanocomposites from spent hen proteins. ACS Omega 2019, 4, 3772–3781. [Google Scholar] [CrossRef] [Green Version]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C 2018, 90, 446–453. [Google Scholar] [CrossRef]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UVA-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]
- Yu, W.; Field, C.J.; Wu, J. A spent hen muscle protein hydrolysate: A potential IL-10 stimulator in a murine model. Food Funct. 2018, 9, 4714–4719. [Google Scholar] [CrossRef]
- Hong, H.; Chaplot, S.; Chalamaiah, M.; Roy, B.C.; Bruce, H.L.; Wu, J. Removing cross-linked telopeptides enhances the production of low-molecular-weight collagen peptides from spent hens. J. Agric. Food Chem. 2017, 65, 7491–7499. [Google Scholar] [CrossRef]
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Well. 2018, 7, 138–147. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Girgih, A.T.; Mohan, A.; Gong, M.; Malomo, S.A.; Aluko, R.E. Antihypertensive and bovine plasma oxidation-inhibitory activities of spent hen meat protein hydrolysates. J. Food Biochem. 2017, 41, e12378. [Google Scholar] [CrossRef]
- Hong, H.; Roy, B.C.; Chalamaiah, M.; Bruce, H.L.; Wu, J. Pretreatment with formic acid enhances the production of small peptides from highly cross-linked collagen of spent hens. Food Chem. 2018, 258, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Howard, A. Meat proteome as source of functional biopeptides. Food Res. Int. 2013, 54, 1021–1032. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.-C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Fan, H.; Liao, W.; Wu, J. Molecular interactions, bioavailability and cellular mechanisms of angiotensin converting enzyme inhibitory (ACEi) peptides. J. Food Biochem. 2018, 43, e12572. [Google Scholar] [CrossRef] [Green Version]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Liao, W.; Jiang, X.; Wu, J. Identification and characterization of gastrointestinal-resistant angiotensin-converting enzyme inhibitory peptides from egg white proteins. J. Agric. Food Chem. 2019, 67, 7147–7156. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Wu, J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) inhibits angiotensin II-stimulated migration of vascular smooth muscle cells via angiotensin type I receptor. J. Agric. Food Chem. 2018, 66, 5133–5138. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, H.; Yu, W.; Hong, H.; Wu, J. Transport study of egg derived antihypertensive peptides (LKP and IQW) using Caco-2 and HT29 co-culture monolayers. J. Agric. Food Chem. 2017, 65, 7406–7414. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory effects of a pea-derived peptide Leu-Arg-Trp (LRW) on dysfunction of rat aortic vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, C.-K.; Shin, H.-K. Utilization of bovine blood plasma proteins for the production of angiotensin I converting enzyme inhibitory peptides. Process. Biochem. 2000, 36, 65–71. [Google Scholar] [CrossRef]
- Gu, Y.; Majumder, K.; Wu, J. QSAR-aided in silico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Res. Int. 2011, 44, 2465–2474. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, M.I.; Butt, M.S.; Riaz, F. Characterization of peptides fractions produced through enzymatic hydrolysis of meat byproducts for their antihypertensive and antioxidant activities. Pak. J. Agric. Sci. 2020, 57, 545–551. [Google Scholar]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Peptides with potential cardioprotective effects derived from dry-cured ham byproducts. J. Agric. Food Chem. 2019, 67, 1115–1126. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, Y.-S.; Kim, S.-E.; Kim, E.-K.; Hwang, J.-W.; Park, T.-K.; Kim, B.K.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J. Purification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from an enzymatic hydrolysate of duck skin byproducts. J. Agric. Food Chem. 2012, 60, 10035–10040. [Google Scholar] [CrossRef]
- Ren, Y.; Wan, D.-G.; Lu, X.-M.; Chen, L.; Zhang, T.-E.; Guo, J.-L. Isolation and characterization of angiotensin I-converting enzyme inhibitor peptides derived from porcine hemoglobin. Sci. Res. Essays 2011, 6, 6262–6269. [Google Scholar]
- Cheng, F.Y.; Liu, Y.T.; Wan, T.C.; Lin, L.C.; Sakata, R. The development of angiotensin I-converting enzyme inhibitor derived from chicken bone protein. Anim. Sci. J. 2008, 79, 122–128. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Sabikun, N.; Ismail, I.; Joo, S.-T. Comparison of meat quality characteristics of wet-and dry-aging pork belly and shoulder blade. Korean J. Food Sci. 2018, 38, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Kaur, L.; Astruc, T.; Vénien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High pressure processing of meat: Effects on ultrastructure and protein digestibility. Food Funct. 2016, 7, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B. We have contact: Endothelial cell-smooth muscle cell interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernanz, R.; Martínez-Revelles, S.; Palacios, R.; Martin, A.; Cachofeiro, V.; Aguado, A.; Garcia-Redondo, L.; Barrus, M.; De Batista, P.; Briones, A. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br. J. Pharmacol. 2015, 172, 3159–3176. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing—Implications in hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg white-derived tripeptide IRW (Ile-Arg-Trp) is an activator of angiotensin converting enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Majumder, K.; Liang, G.X.; Chen, Y.H.; Guan, L.L.; Davidge, S.T.; Wu, J.P. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 1735–1744. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wang, J.; Pan, H.; Wu, H.; Ren, D.; Lu, J. Effects of IQP, VEP and Spirulina platensis hydrolysates on the local kidney renin angiotensin system in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 16, 8485–8492. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.-H.; Zhang, W.-G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Mats, L.; Liu, R.; Deng, Z.; Mine, Y.; Tsao, R. Anti-inflammatory effect and cellular uptake mechanism of peptides from common bean (Phaseolus vulga L.) milk and yogurts in Caco-2 mono-and Caco-2/EA. hy926 co-culture models. J. Agric. Food Chem. 2019, 67, 8370–8381. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Esfandi, R.; Willmore, W.G.; Tsopmo, A. Antioxidant activity of oat proteins derived peptides in stressed hepatic HepG2 cells. Antioxidants 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, G.; ud Din, J.; Zhao, F.; Liu, X. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, J. Bovine lactoferrin-derived ACE inhibitory tripeptide LRP also shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2016, 25, 375–384. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Tech. 2019, 86, 399–411. [Google Scholar] [CrossRef]





| Enzymes | Hydrolysis Yield (%) | Protein Content (%) | DH (%) | ACE Inhibition (%) * |
|---|---|---|---|---|
| (IC50, μg/mL) | ||||
| Alcalase | 79.8 ± 4.0 ab | 70.0 ± 1.0 c | 22.1 ± 2.3 b | 52.5 ± 1.1 (57 ± 2.4) d |
| Protex 6L | 65.0 ± 5.6 cd | 71.8 ± 2.4 bc | 17.3 ± 0.9 c | 42.8 ± 2.2 (79 ± 1.7) e |
| Protease S | 47.8 ± 4.2 e | 73.6 ± 2.1 bc | 12.2 ± 1.3 d | 59.8 ± 1.0 (39 ± 0.5) c |
| Thermoase | 86.5 ± 1.4 a | 79.2 ± 1.2 a | 21.9 ± 0.1 b | 64.8 ± 0.9 (30 ± 1.4) b |
| Trypsin | 44.4 ± 0.1 e | 83.4 ± 2.2 a | 11.2 ± 0.0 d | 18.6 ± 2.7 (189 ± 4.7) g |
| Protease M | 64.5 ± 3.9 cd | 81.0 ± 0.7 a | 32.7 ± 0.2 a | 39.1 ± 0.2 (118 ± 5.0) f |
| Pepsin | 68.6 ± 0.8 c | 73.6 ± 1.5 bc | 12.2 ± 0.7 d | 70.8 ± 0.2 (23 ± 0.9) a |
| Protex 50FP | 58.4 ± 2.1 d | 74.7 ± 0.6 b | 16.3 ± 2.2 c | 52.7 ± 0.2 (50 ± 1.6) d |
| Protex 26L | 76.4 ± 1.5 b | 73.8 ± 2.2 bc | 20.6 ± 0.6 b | 69.5 ± 0.7 (24 ± 1.5) a |
| Samples | A7r5 Cells | EA.hy926 Cells | ||
|---|---|---|---|---|
| ACE2 | Oxidative Stress | ICAM-1 | VCAM-1 | |
| Untreated | Ang II (+) | TNFα (+) | TNFα (+) | |
| SPH-T | 1.82 ± 0.36 * | 0.73 ± 0.06 * | 1.02 ± 0.08 | 0.80 ± 0.03 * |
| SPH-P | 1.01 ± 0.17 | 0.77 ± 0.07 * | 1.05 ± 0.13 | 0.73 ± 0.04 * |
| SPH-26L | 0.98 ± 0.08 | 0.67 ± 0.13 * | 1.10 ± 0.04 | 0.74 ± 0.07 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Yu, W.; Liao, W.; Wu, J. Spent Hen Protein Hydrolysate with Good Gastrointestinal Stability and Permeability in Caco-2 Cells Shows Antihypertensive Activity in SHR. Foods 2020, 9, 1384. https://doi.org/10.3390/foods9101384
Fan H, Yu W, Liao W, Wu J. Spent Hen Protein Hydrolysate with Good Gastrointestinal Stability and Permeability in Caco-2 Cells Shows Antihypertensive Activity in SHR. Foods. 2020; 9(10):1384. https://doi.org/10.3390/foods9101384
Chicago/Turabian StyleFan, Hongbing, Wenlin Yu, Wang Liao, and Jianping Wu. 2020. "Spent Hen Protein Hydrolysate with Good Gastrointestinal Stability and Permeability in Caco-2 Cells Shows Antihypertensive Activity in SHR" Foods 9, no. 10: 1384. https://doi.org/10.3390/foods9101384
APA StyleFan, H., Yu, W., Liao, W., & Wu, J. (2020). Spent Hen Protein Hydrolysate with Good Gastrointestinal Stability and Permeability in Caco-2 Cells Shows Antihypertensive Activity in SHR. Foods, 9(10), 1384. https://doi.org/10.3390/foods9101384