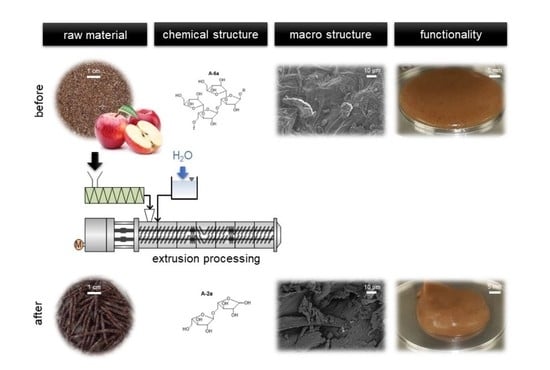
3.3. Impact of Extrusion on Molecular Structures
The four samples 100-42-200, 100-22-200, 100-22-450 and 100-22-700 (see
Section 3.2.5) were used to analyze the macroscopic and molecular structures of apple pomace before and after extrusion. To describe extrusion-based changes independently of the particle size and to characterize the application-relevant material, a detailed structural characterization was performed using the entire ground fraction as described in
Section 3.2.5. However, to roughly check whether sieving has an impact on the polymer chemical composition, the monosaccharide composition of the polysaccharides of the sieved fraction (particle size of 0.14–0.28 mm) was also determined (after both sulfuric acid hydrolysis and methanolysis). The data (Supporting Information,
Table A1) did not indicate major compositional differences between the sieved and the non-sieved samples, disapproving the possibility that, for example, starch had been systematically sieved out due to the formation of smaller particles during grinding.
Protein, fat, ash content. The methods and data of the analysis of protein, fat and ash are given in the Supporting Information (
Table A2) simply to characterize the raw material and extruded products. Below, we will focus on carbohydrates.
Free mono- and disaccharides. Because low-molecular weight compounds may also contribute to the physicochemical properties of the pomace, mono- and disaccharides were analyzed. Glucose, fructose, sucrose and maltose were detected and quantified as free mono- and disaccharides (Supporting Information,
Table A3). The glucose content (2.8 ± 0.3 g/100 g dry matter (DM)) did not change significantly during extrusion. In contrast, and as expected from the literature [
58], the fructose content decreased with increasing thermo-mechanical treatment (from 8.8 ± 0.3 g/100 g DM to 3.4 ± 0.1 g/100 g DM (100-22-700)). Also, the sucrose content decreased from 2.3 ± 0.4 g/100 g DM to 0.7 ± 0.5 g/100 g DM (100-22-700), and maltose contents decreased independently of the extrusion conditions from 1.4 ± 0.3 g/100 g DM to below the limit of quantitation.
Starch. Depending on the stage of maturity, apples and thus apple pomace contain significant amounts of starch. The raw material contained 11.6 ± 0.7 g starch/100 g DM. Starch contents decreased to 8.5–9.2 g/100 g DM during extrusion (
Table A4, Supporting Information) with the decline being widely independent of the extrusion conditions.
Dietary fiber composition. Insoluble dietary fiber contents decreased independently of the extrusion conditions (
Table 1). Considering extrusion conditions using 22% water, soluble dietary fiber contents increased with increasing thermo-mechanical treatment. This trend was consistent with WSI data, although it needs to be considered that dietary fiber data exclude mono- and disaccharides, starch and protein, which are included in the WSI assay. Low-molecular-weight soluble dietary fiber was only formed at a screw speed of 700 min
−1, which can easily be explained by extensive polymer degradation under high thermo-mechanical stress.
Unlike in the case of the WSI data and also in disagreement with the applied thermo-mechanical stress, the soluble dietary fiber content was higher for the 100-42-200 sample than for the 100-22-200 sample. A simple explanation is difficult to find; however, Maillard reaction products that are partially captured using the dietary fiber methodology might be at least one factor contributing to this unexpected finding.
The dietary fiber contents of apple pomace have been determined in the past [
7,
59]. According to the literature, insoluble and soluble fiber contents are dependent on the variety of apples (insoluble dietary fiber ranges between 33–67%, soluble fiber between 3–14%). The data analyzed here are within the range of the values found in the literature. Besides taking into account the variety, it needs to be considered that fiber contents and composition also depend on tissue maturity and changes during storage [
25,
60]. The influence of different extrusion parameters on fiber contents has been previously studied by Hwang and coworkers [
32], who also found decreasing insoluble dietary fiber and increasing soluble fiber contents due to extrusion.
Polysaccharide characterization. Dietary fiber data indicate that thermo-mechanical treatment influences the structure of fiber polysaccharides, potentially going along with polymer solubilization and more or less specific degradation. This hypothesis is supported by data from the molecular weight distribution analysis. This analysis was used to assess the extent of structural changes of the soluble dietary fiber fraction rather than determining the exact molecular weight.
Figure 9 shows that the molecular weight distribution became broader with increasing thermo-mechanical treatment, suggesting that large polymers had been (partially) broken down, thus backing up the dietary fiber composition data described above.
The monosaccharide compositions of insoluble fiber polysaccharides of raw material and extruded samples were determined after sulfuric acid hydrolysis (
Table 2). Glucose was the main monomer (43.4 mol%) in the raw material, followed by arabinose (17.3 mol%), galacturonic acid (11.9 mol%), xylose (11.0 mol%) and galactose (10.1 mol%). The main portion of glucose could be attributed to cellulose, as well as being part of the hemicellulose xyloglucan. Xyloglucans, xylogalacturonans and secondary cell wall xylans are potential sources of xylose. Mannans are quantitatively less important in apple pomace insoluble fiber. The remaining monosaccharides could largely be assigned to pectic polymers (in total about 42 mol%) with arabinose and galactose being the most important units of rhamnogalacturonan I side chains, and galacturonic acid being a constituent of all pectic polysaccharides. It should be noted, however, that uronic acids are underestimated by using sulfuric acid hydrolysis. Overall, the insoluble dietary fiber monosaccharide composition was very similar to the recently published data of the composition of non-starch polysaccharides from apples after sulfuric acid hydrolysis [
25].
The data of extruded samples indicate that insoluble fiber arabinose contents decreased by up to 7% (absolute) with increasing thermo-mechanical treatment, which is in agreement with the data published by Hwang et al. [
32]. Also, the portion of galacturonic acid decreased in correlation with an increase in glucose and xylose.
The monosaccharide composition of apple pomace soluble dietary fiber polysaccharides was determined after methanolysis (
Table 3). Not surprisingly, the constituents of pectic polysaccharides clearly dominated the soluble fiber fraction. Arabinose was the main monosaccharide (42.0 mol%), followed by galacturonic acid (32.9 mol%) and galactose (8.8 mol%). Similarly to the insoluble dietary fiber polysaccharides, the amount of arabinose decreased by applying thermo-mechanical stress. However, unlike in the case of insoluble fiber, comparably weak thermo-mechanical treatment already had a distinct effect on arabinose portions, which were only slightly decreased with harsher treatment. Galactose and xylose containing polysaccharides appeared to be more stable, as indicated by the growing portions with harsher treatment.
However, these data and additional structural data have to be interpreted keeping in mind two mechanisms of how the compositions of the soluble and insoluble dietary fiber preparations can be altered: 1) solubilization of formerly insoluble polysaccharides, shifting them into the soluble fiber fraction, and 2) actual decomposition of specific structural units in the insoluble or soluble fiber fraction. Also, carbohydrates may be degraded or converted into Maillard products, thus escaping our analyses. As these mechanisms occur simultaneously, definite conclusions are sometimes difficult to draw.
The low–molecular-weight soluble dietary fiber that was produced in the 100-22-700 was mainly composed of arabinose and galactose (35.9% and 18.2%), respectively, and, surprisingly, glucose (41.8%). Xylose (0.9%) and mannose (3.1%) were only minor constituents.
Methylation analysis of the insoluble dietary fiber fraction showed that glucose was mainly a constituent of cellulose (1,4-linked) with lower portions being involved in xyloglucans (1,4,6-linked; 1,4-linked) (
Table 4). 1,2-Linked xylopyranose units may have occurred as side chains of xyloglucans. Terminal xylopyranose could be assigned to both xyloglucans and xylans. However, low amounts of 1,4-linked xylopyranose (1.9 mol%) suggested small amounts of xylans as secondary cell wall components only. The identification of mannose after sulfuric acid hydrolysis and low levels of 1,4-linked mannopyranose and 1,4,6-linked mannopyranose demonstrated the existence of (partially branched) mannans in the insoluble fiber fraction. Methylation analysis confirmed that arabinose was mostly integrated in arabinans (1,5-linked) with branches in position
O-2,
O-3, or in both positions (1,2,5-, 1,3,5- and 1,2,3,5- linked arabinofuranose units). Branches in position
O-3 dominated over branches in position
O-2, as previously demonstrated by Wefers, Flörchinger et al. [
25], and doubly substituted arabinose units were quite common. Galactans were mostly unsubstituted (1,4-linked). Methylation analysis data demonstrated the impact of extrusion on insoluble fiber polysaccharides, confirming assumptions made from the analysis of the monosaccharide composition. Changes were most obvious in samples treated at a screw speed of 700 min
−1: terminal arabinofuranose and 1,3,5-linked arabinofuranose portions were distinctly decreased. Also, doubly substituted arabinan units were decreased using the harshest extrusion conditions. Comparable observations were also made by Schmid et al. [
61], who studied thermo-mechanically treated aronia pomace. Galactans, which are often difficult to fully capture using methylation analysis, did not show a clear extrusion affected behavior.
To confirm the methylation analysis data on arabinans and galactans, an arabinan and galactan screening was performed according to the method described by Wefers, Bunzel et al. [
45]. Using
endo-arabinanases and
endo-galactanases, linear α-(1→5)-linked regions within the arabinan backbone and β-(1→4)-linked regions within the galactan backbone were cleaved, releasing oligomeric units (
Table 5, for structures see
Figure A1 in the Supporting Information). The results did not fully support methylation analysis data with regard to
O-3-linked structural units (as mostly reflected by compound A-4a), but also showed a decrease in doubly substituted arabinan structural elements (as reflected by A5a) and generally demonstrated that arabinan complexity was reduced with the application of thermo-mechanical stress. The galactan screening assay confirmed the existence of galactans but did not reveal additional structural data. Xylans, as reflected by 1,4-linked xylopyranose units in the methylation analysis, appeared to be more stable during extrusion and were enriched in the 100-22-700 sample.
Methylation analysis of the soluble dietary fiber fraction confirmed the dominance of the arabinan building blocks among the neutral monosaccharides. Just as seen for the insoluble fiber fraction, branches in position
O-3 dominated over branches in position
O-2, and doubly substituted arabinose units were shown to be important structural features in apple pomace soluble fiber. Most distinct effects were seen when the harshest extrusion conditions were applied (100-22-700). Again, the portion of terminal arabinose units was strongly reduced; however, the effect on 1,3,5- (and 1,2,3,5-) linked arabinose units was negligible. Although the changes were minor, xyloglucans may have been enriched in this extruded sample as compared to the raw material. Application of the enzymatic arabinan and galactan profiling assay confirmed the existence of the structural units determined by using methylation analysis (
Table A5 and
Table A6, Supporting Information) but did not reveal any additional information with respect to the stability of structural units against thermo-mechanical stress.
The degree of esterification of polymer-bound galacturonic acid affects gel formation depending on the gel formation mechanism used. Our data suggest that thermo-mechanical stress reduced the degree of esterification, as clearly demonstrated for the insoluble dietary fiber fraction: the degree of esterification decreased with increasing screw speed from 50% (raw material) to 15% (100-22-700). Comparable behavior was observed for soluble dietary fiber pectic polysaccharides. Modifications with acetates appeared to be more stable than with methyl esters; however, a reduction of the acetylation degree in the extruded samples was observed for the insoluble dietary fiber pectic polysaccharides.