Head-to-Head Study of Developmental Neurotoxicity and Resultant Phenotype in Rats: α-Hexabromocyclododecane versus Valproic Acid, a Recognized Model of Reference for Autism Spectrum Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Doses and Protocol of Exposure
2.4. Behavioural Testing
2.4.1. Maternal Behaviour and Offspring Monitoring
2.4.2. Neuromotor Development Assessment
2.5. Histochemical Measurement of Cytochrome Oxidase Activity
2.6. Western Blot
2.7. Statistical Analysis
3. Results and Discussion
3.1. Gestational Outcomes and Maternal Behaviour
3.2. Effects of α-HBCDD and VPA Exposure on Male Offspring Weight
3.3. Effects of α-HBCDD and VPA Exposure on Anogenital Distance (AGD)
3.4. Effects of α-HBCDD and VPA Exposure on Motor Development
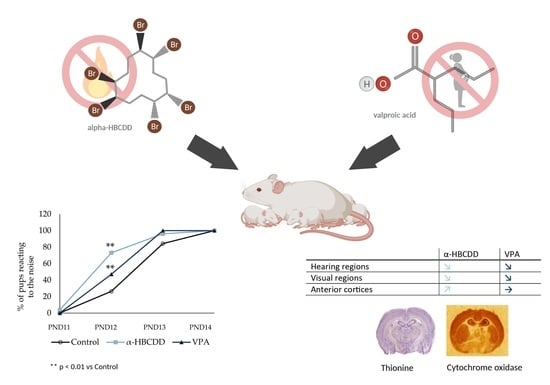
3.5. Effects of α-HBCDD and VPA Exposure on Auditory Perception
3.6. Neuroglia and Synaptic Plasticity in the Cortex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bossu, J.L.; Roux, S. The valproate model of autism. Med. Sci. 2019, 35, 236–243. [Google Scholar]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, D.Q.; Stevens, H.E.; Margolis, K.G.; Van De Water, J. Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Curr. Pharm. Des. 2019, 25, 4331–4343. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Schroeder, H.; Olivier, J.-L.; Turner, J.D. Epigenetic and Neurological Impairments Associated with Early Life Exposure to Persistent Organic Pollutants. Int. J. Genom. 2019, 2019, 2085496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belardo, A.; Gevi, F.; Zolla, L. The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children. J. Nutr. Biochem. 2019, 70, 38–46. [Google Scholar] [CrossRef]
- Robea, M.-A.; Luca, A.-C.; Ciobica, A. Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina 2020, 56, 245. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Schmidt, B.; Cain, U.; Lemcke, N.; Foley, J.T.; Peck, R.; Clemons, T.; Reynolds, A.; Johnson, C.; et al. Nutrient Intake From Food in Children With Autism. Pediatrics 2012, 130, S145–S153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbott, E.O.; Arena, V.C.; Rager, J.R.; Clougherty, J.E.; Michanowicz, D.R.; Sharma, R.K.; Stacy, S.L. Fine particulate matter and the risk of autism spectrum disorder. Environ. Res. 2015, 140, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Dhulkifle, H.; Agouni, A.; Zeidan, A.; Al-Kuwari, M.S.; Parray, A.; Tolefat, M.; Korashy, H.M. Influence of the Aryl Hydrocarbon Receptor Activating Environmental Pollutants on Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 9258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, B.L.; Yi, M.J.; Zhang, F.H. Association of exposure to polycyclic aromatic hydrocarbons during pregnancy with autism spectrum disorder-related behaviors in toddlers: A birth cohort study. Chin. J. Contemp. Pediatrics 2019, 21, 332–336. [Google Scholar]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.A.; Billock, V.A. Developmental neurotoxicity and autism: A potential link between indoor neuroactive pollutants and the curious birth order risk factor. Int. J. Dev. Neurosci. 2017, 62, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Schroeder, H.; Emond, C.; Turner, J.D.; Lichtfouse, E.; Grova, N. Brominated flame retardants, a cornelian dilemma. Environ. Chem. Lett. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ongono, J.S.; Béranger, R.; Baghdadli, A.; Mortamais, M. Pesticides used in Europe and autism spectrum disorder risk: Can novel exposure hypotheses be formulated beyond organophosphates, organochlorines, pyrethroids and carbamates?-A systematic review. Environ. Res. 2020, 187, 109646. [Google Scholar] [CrossRef]
- Kim, U.J.; Oh, J.E. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ. Pollut. 2014, 184, 193–200. [Google Scholar] [CrossRef]
- Anisuzzaman, S.; Whalen, M.M. Tetrabromobisphenol A and hexabromocyclododecane alter secretion of IL-1β from human immune cells. J. Immunotoxicol. 2015, 13, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, I.; Serchi, T.; Cambier, S.; Diepenbroek, C.; Renaut, J.; Van der Berg, J.; Kwadijk, C.; Gutleb, A.; Rijntjes, E.; Murk, A. Hexabromocyclododecane (HBCD) induced changes in the liver proteome of eu- and hypothyroid female rats. Toxicol. Lett. 2016, 245, 40–51. [Google Scholar] [CrossRef]
- Fernandes, S.B.; Grova, N.; Roth, S.; Duca, R.C.; Godderis, L.; Guebels, P.; Mériaux, S.B.; Lumley, A.I.; Bouillaud-Kremarik, P.; Ernens, I.; et al. N6-Methyladenine in Eukaryotic DNA: Tissue Distribution, Early Embryo Development, and Neuronal Toxicity. Front. Genet. 2021, 12, 657171. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.; Olry, J.C.; Cariou, R.; Dervilly-Pinel, G.; Le Bizec, B.; Travel, A.; Jondrevillea, C.; Schroedera, H. Short-term effects of a perinatal exposure to the HBCDD alpha-isomer in rats: Assessment of early motor and sensory development, spontaneous locomotor activity and anxiety in pups. Neurotoxicol. Teratol. 2015, 52, 170–180. [Google Scholar] [CrossRef]
- Schiavi, S.; Iezzi, D.; Manduca, A.; Leone, S.; Melancia, F.; Carbone, C.; Petrella, M.; Mannaioni, G.; Masi, A.; Trezza, V. Reward-Related Behavioral, Neurochemical and Electrophysiological Changes in a Rat Model of Autism Based on Prenatal Exposure to Valproic Acid. Front. Cell. Neurosci. 2019, 13, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appenzeller, B.M.R.; Hardy, E.M.; Grova, N.; Chata, C.; Faÿs, F.; Briand, O.; Schroeder, H.; Duca, R.-C. Hair analysis for the biomonitoring of pesticide exposure: Comparison with blood and urine in a rat model. Arch. Toxicol. 2016, 91, 2813–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crépeaux, G.; Grova, N.; Bouillaud-Kremarik, P.; Sikhayeva, N.; Salquèbre, G.; Rychen, G.; Soulimani, R.; Appenzeller, B.; Schroeder, H. Short-term effects of a perinatal exposure to a 16 polycyclic aromatic hydrocarbon mixture in rats: Assessment of early motor and sensorial development and cerebral cytochrome oxidase activity in pups. NeuroToxicology 2014, 43, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; p. 456. [Google Scholar]
- Favre, M.R.; Barkat, T.R.; LaMendola, D.; Khazen, G.; Markram, H.; Markram, K. General developmental health in the VPA-rat model of autism. Front. Behav. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- DeGroote, S.; Hunting, D.; Sébire, G.; Takser, L. Autistic-like traits in Lewis rats exposed perinatally to a mixture of common endocrine disruptors. Endocr. Disruptors 2014, 2, e976123. [Google Scholar] [CrossRef] [Green Version]
- Saegusa, Y.; Fujimoto, H.; Woo, G.-H.; Inoue, K.; Takahashi, M.; Mitsumori, K.; Hirose, M.; Nishikawa, A.; Shibutani, M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod. Toxicol. 2009, 28, 456–467. [Google Scholar] [CrossRef]
- Miller-Rhodes, P.; Popescu, M.; Goeke, C.; Tirabassi, T.; Johnson, L.; Markowski, V.P. Prenatal exposure to the brominated flame retardant hexabromocyclododecane (HBCD) impairs measures of sustained attention and increases age-related morbidity in the Long–Evans rat. Neurotoxicol. Teratol. 2014, 45, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Fujii, S.; Hirata-Koizumi, M.; Matsumoto, M. Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats. Reprod. Toxicol. 2008, 25, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Ruhela, R.K.; Soni, S.; Sarma, P.; Prakash, A.; Medhi, B. Negative geotaxis: An early age behavioral hallmark to VPA rat model of autism. Ann. Neurosci. 2019, 26, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ding, R.; Song, Y.; Wang, J.; Zhang, C.; Han, S.; Han, J.; Zhang, R. Transcutaneous Electrical Acupoint Stimulation in Early Life Changes Synaptic Plasticity and Improves Symptoms in a Valproic Acid-Induced Rat Model of Autism. Neural Plast. 2020, 2020, 8832694. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Breyer, B.N.; Eisenberg, M.L.; Baskin, L.S. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr. Urol. Rep. 2008, 9, 137–142. [Google Scholar] [CrossRef]
- Schwartz, C.L.; Christiansen, S.; Vinggaard, A.M.; Axelstad, M.; Hass, U.; Svingen, T. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch. Toxicol. 2019, 93, 253–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallavan, R.H.; Holson, J.F.; Stump, D.G.; Knapp, J.F.; Reynolds, V.L. Interpreting the toxicologic significance of alterations in anogenital distance: Potential for confounding effects of progeny body weights. Reprod. Toxicol. 1999, 13, 383–390. [Google Scholar] [CrossRef]
- Källén, B. Valproic acid is known to cause hypospadias in man but does not reduce anogenital distance or causes hypospadias in rats. Basic Clin. Pharmacol. Toxicol. 2004, 94, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.G.; Lefèvre, P.L.; Ernest, S.R.; Wade, M.; Ma, Y.-Q.; Rawn, D.F.; Gaertner, D.W.; Robaire, B.; Hales, B.F. Exposure to an environmentally relevant mixture of brominated flame retardants affects fetal development in Sprague-Dawley rats. Toxicology 2014, 320, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Wang, Y.; Li, Y.; Chen, D.; Yang, F.; Wang, S. A Developmental Study of Abnormal Behaviors and Altered GABAergic Signaling in the VPA-Treated Rat Model of Autism. Front. Behav. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, B.; Lopez, B.R.; Heimerl, S. A Comparison of Motor Delays in Young Children: Autism Spectrum Disorder, Developmental Delay, and Developmental Concerns. J. Autism Dev. Disord. 2007, 37, 321–328. [Google Scholar] [CrossRef]
- Hazen, E.P.; Stornelli, J.L.; O’Rourke, J.A.; Koesterer, K.; McDougle, C.J. Sensory Symptoms in Autism Spectrum Disorders. Harv. Rev. Psychiatry 2014, 22, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitoglou, M.; Ververi, A.; Antoniadis, A.; Zafeiriou, D.I. Childhood Autism and Auditory System Abnormalities. Pediatr. Neurol. 2010, 42, 309–314. [Google Scholar] [CrossRef]
- Codagnone, M.; Podestá, M.F.; Uccelli, N.A.; Reinés, A. Differential Local Connectivity and Neuroinflammation Profiles in the Medial Prefrontal Cortex and Hippocampus in the Valproic Acid Rat Model of Autism. Dev. Neurosci. 2015, 37, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Bronzuoli, M.R.; Facchinetti, R.; Ingrassia, D.; Sarvadio, M.; Schiavi, S.; Steardo, L.; Verkhratsky, A.; Trezza, V.; Scuderi, C. Neuroglia in the autistic brain: Evidence from a preclinical model. Mol. Autism 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Traetta, M.E.; Uccelli, N.A.; Zárate, S.C.; Cuautle, D.G.; Ramos, A.J.; Reinés, A. Long-Lasting Changes in Glial Cells Isolated from Rats Subjected to the Valproic Acid Model of Autism Spectrum Disorder. Front. Pharmacol. 2021, 12, 707859. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Park, J.H.; Kim, H.J.; Jeon, S.J.; Pena, I.C.D.; Han, S.-H.; Cheong, J.H.; et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neurochem. 2013, 124, 832–843. [Google Scholar] [CrossRef]
- Antonucci, F.; Corradini, I.; Fossati, G.; Tomasoni, R.; Menna, E.; Matteoli, M. SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions. Front. Synaptic Neurosci. 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenart, J.; Bratek, E.; Lazarewicz, J.W.; Zieminska, E. Changes in the Expression of SNAP-25 Protein in the Brain of Juvenile Rats in Two Models of Autism. J. Mol. Neurosci. 2020, 70, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Zieminska, E.; Lenart, J.; Lazarewicz, J.W. Select putative neurodevelopmental toxins modify SNAP-25 expression in primary cultures of rat cerebellar granule cells. Toxicology 2016, 370, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [Green Version]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2020, 44, 227–240. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Abramson, I.; Semendeferi, K.; Courchesne, E.; Everall, I.P. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 2012, 1456, 72–81. [Google Scholar] [CrossRef]
- Araque, A.; Navarrete, M. Glial cells in neuronal network function. Philosophical transactions of the Royal Society of London. Series B. Biol. Sci. 2010, 365, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Madia, F.; Giordano, G.; Fattori, V.; Vitalone, A.; Branchi, I.; Capone, F.; Costa, L.G. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004, 154, 11–21. [Google Scholar] [CrossRef] [PubMed]





| Control | α–HBCDD | VPA | Factor | Statistical Index | |||
|---|---|---|---|---|---|---|---|
| (100 ng/kg/day) | (600 mg/kg) | (df) | p | ||||
| Gestational outcome | |||||||
| Number of females included | 6 | 6 | 6 | - | - | - | |
| Reproductive success | 5/6 (83.3%) | 5/6 (83.3%) | 6/6 (100%) | Chi2 | 1.125 (df = 2) | 0.570 | |
| Number of surviving females | 5/5 (100%) | 5/5 (100%) | 4/6 (66.7%) | Chi2 | 3.810 (df = 2) | 0.149 | |
| Gestation length (days) | 22 (22–23) | 22 (22–23) | 23 (23–23) | K–W | 3.900 (df = 2) | 0.142 | |
| Total number of pups | 7 (6–12) | 10 (10–12) | 10 (10–10) | K–W | 0.511 (df = 2) | 0.775 | |
| Total number of males | 4 (4–7) | 6 (5–6) | 4 (4–6) | K–W | 0.473 (df = 2) | 0.789 | |
| Total number of females | 3 (3–4) | 4 (4–5) | 6 (4–6) | K–W | 2.493 (df = 2) | 0.287 | |
| Sex ratio/litter | 1.33 (1.17–2.00) | 1.40 (1.33–1.50) | 0.83 (0.62–1.67) | K–W | 0.748 (df = 2) | 0.688 | |
| Nest building (PND9) (%) | |||||||
| 1 h | No building | 25 | 100 | 25 | Chi2 | n.a. (df = 4) | p < 0.05 |
| Partial building | 75 | 0 | 75 | ||||
| Full building | 0 | 0 | 0 | ||||
| 2 h | No building | 25 | 100 | 25 | Chi2 | n.a. (df = 4) | p < 0.05 |
| Partial building | 75 | 0 | 75 | ||||
| Full building | 0 | 0 | 0 | ||||
| 24 h | No building | 0 | 0 | 0 | Chi2 | n.a. (df = 4) | 1.000 |
| Partial building | 25 | 20 | 0 | ||||
| Full building | 75 | 80 | 100 | ||||
| Pup retrieving (% of Yes) | |||||||
| PND4 | 50 | 40 | 50 | Chi2 | 0.124 (df = 2) | 0.940 | |
| PND7 | 75 | 40 | 50 | Chi2 | 1.130 (df = 2) | 0.568 | |
| PND10 | 100 | 80 | 25 | Chi2 | 5.724 (df = 2) | p < 0.05 | |
| Selected Brain Regions | Control | α-HBCDD | VPA | F (2,10) | p | ||
|---|---|---|---|---|---|---|---|
| (100 ng/kg/day) | (600 mg/kg) | ||||||
| Hearing | |||||||
| Auditory Cortex | 39.8 ± 1.1 | 40.0 ± 1.3 | 32.3 ± 3.1 | ♦# | 4.973 | 0.032 | |
| Medial geniculate nucleus | 37.0 ± 0.8 | 38.0 ± 0.9 | 32.0 ± 1.7 | *# | 7.856 | 0.009 | |
| Inferior colliculus | 38.4 ± 0.5 | 33.1 ± 2.1 | 29.0 ± 1.7 | * | 6.960 | 0.013 | |
| Superior olive | 31.7 ± 0.8 | 34.4 ± 2.2 | 20.3 ± 10.4 | 2.323 | 0.154 | ||
| Lateral lemniscus | 30.8 ± 1.2 | 22.9 ± 3.2 | 19.5 ± 2.5 | * | 5.467 | 0.032 | |
| Vision | |||||||
| Visual Cortex | 36.2 ± 1.3 | 32.4 ± 0.9 | 30.0 ± 1.5 | * | 6.541 | 0.018 | |
| Superior colliculus | 30.6 ± 0.4 | 33.2 ± 2.2 | 32.1 ± 0.6 | 0.731 | 0.506 | ||
| Olfaction | |||||||
| Olfactory tubercle | 34.9 ± 0.8 | 35.8 ± 0.5 | 35.5 ± 0.9 | 0.346 | 0.717 | ||
| Piriform cortex | 36.2 ± 4.6 | 36.0 ± 2.4 | 35.0 ± 2.0 | 0.051 | 0.951 | ||
| Mammillary Bodies | |||||||
| Lateral core | 28.1 ± 1.8 | 34.3 ± 6.0 | 27.1 ± 3.6 | 1.114 | 0.388 | ||
| Medial nucleus, median part | 29.5 ± 1.9 | 34.7 ± 2.6 | 33.0 ± 1.3 | 1.783 | 0.247 | ||
| Medial nucleus, lateral part | 31.4 ± 2.2 | 35.9 ± 2.8 | 32.3 ± 1.9 | 0.957 | 0.436 | ||
| Anterior Cortices | |||||||
| Cingulate cortex | 28.9 ± 0.4 | 30.4 ± 1.7 | 23.9 ± 1.0 | ♦# | 6.768 | 0.014 | |
| Prelimbic cortex | 28.1 ± 0.4 | 29.9 ± 1.6 | 25.2 ± 1.5 | 3.005 | 0.095 | ||
| Infralimbic cortex | 27.1 ± 0.6 | 27.9 ± 2.0 | 26.7 ± 0.6 | 0.188 | 0.832 | ||
| Frontal cortex | 31.1 ± 0.9 | 35.7 ± 0.9 | * | 30.8 ± 1.0 | # | 9.135 | 0.006 |
| Hippocampus | |||||||
| CA1 | 33.1 ± 1.6 | 33.6 ± 1.8 | 31.3 ± 2.2 | 0.407 | 0.676 | ||
| CA2 | 33.1 ± 1.5 | 36.7 ± 2.3 | 35.2 ± 2.2 | 0.759 | 0.493 | ||
| CA3 | 36.3 ± 0.9 | 39.9 ± 2.4 | 36.6 ± 2.1 | 1.029 | 0.392 | ||
| Dentate gyrus | 33.4 ± 0.4 | 36.1 ± 2.1 | 33.9 ± 1.7 | 0.745 | 0.499 | ||
| Entorhinal cortex | 31.5 ± 1.8 | 34.3 ± 1.3 | 30.1 ± 1.3 | 2.295 | 0.151 | ||
| Amygdala | |||||||
| Central nucleus | 33.2 ± 1.7 | 33.6 ± 2.1 | 31.8 ± 2.0 | 0.234 | 0.796 | ||
| Medial nucleus antero dorsal | 28.8 ± 3.9 | 30.0 ± 3.2 | 29.5 ± 1.6 | 0.041 | 0.960 | ||
| Intercalated nuclei | 32.0 ± 1.7 | 32.6 ± 1.8 | 31.9 ± 2.3 | 0.036 | 0.964 | ||
| Basolateral nucleus | 34.0 ± 2.6 | 34.2 ± 2.3 | 30.4 ± 1.3 | 0.990 | 0.409 | ||
| Basomedial nucleus | 28.6 ± 3.9 | 30.5 ± 3.0 | 27.9 ± 2.1 | 0.213 | 0.813 | ||
| Cerebellum | |||||||
| White matter | 19.2 ± 0.1 | 12.7 ± 2.8 | 18.7 ± 2.1 | 2.911 | 0.101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morel, C.; Christophe, A.; Maguin-Gaté, K.; Paoli, J.; Turner, J.D.; Schroeder, H.; Grova, N. Head-to-Head Study of Developmental Neurotoxicity and Resultant Phenotype in Rats: α-Hexabromocyclododecane versus Valproic Acid, a Recognized Model of Reference for Autism Spectrum Disorders. Toxics 2022, 10, 180. https://doi.org/10.3390/toxics10040180
Morel C, Christophe A, Maguin-Gaté K, Paoli J, Turner JD, Schroeder H, Grova N. Head-to-Head Study of Developmental Neurotoxicity and Resultant Phenotype in Rats: α-Hexabromocyclododecane versus Valproic Acid, a Recognized Model of Reference for Autism Spectrum Disorders. Toxics. 2022; 10(4):180. https://doi.org/10.3390/toxics10040180
Chicago/Turabian StyleMorel, Chloé, Armelle Christophe, Katy Maguin-Gaté, Justine Paoli, Jonathan David Turner, Henri Schroeder, and Nathalie Grova. 2022. "Head-to-Head Study of Developmental Neurotoxicity and Resultant Phenotype in Rats: α-Hexabromocyclododecane versus Valproic Acid, a Recognized Model of Reference for Autism Spectrum Disorders" Toxics 10, no. 4: 180. https://doi.org/10.3390/toxics10040180
APA StyleMorel, C., Christophe, A., Maguin-Gaté, K., Paoli, J., Turner, J. D., Schroeder, H., & Grova, N. (2022). Head-to-Head Study of Developmental Neurotoxicity and Resultant Phenotype in Rats: α-Hexabromocyclododecane versus Valproic Acid, a Recognized Model of Reference for Autism Spectrum Disorders. Toxics, 10(4), 180. https://doi.org/10.3390/toxics10040180