Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Robustness Testing
2.3. Evaluation of Probe Depletion
2.4. Statistical Analysis
3. Results
3.1. Cause and Effect Analysis
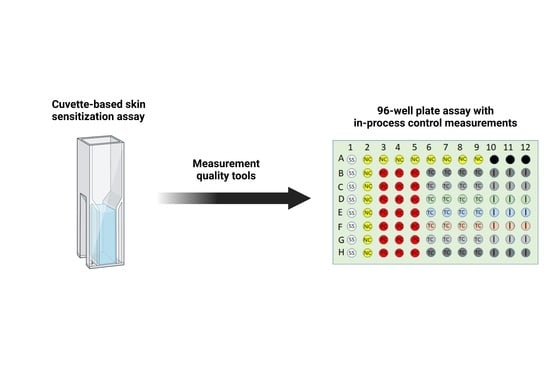
3.2. Plate Layout and In-Process Control Measurements
3.3. Robustness Testing and Protocol Design
3.4. Control Charting Results
3.5. Test Substance Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabian, E.; Oesch, F.; Ott, K.; Landsiedel, R.; van Ravenzwaay, B. A protocol to determine dermal absorption of xenobiotica through human skin in vitro. Arch. Toxicol. 2017, 91, 1497–1511. [Google Scholar] [CrossRef]
- Guth, K.; Schaefer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. In Vitro 2015, 29, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Kolle, S.N.; van Ravenzwaay, B.; Landsiedel, R. Regulatory accepted but out of domain: In vitro skin irritation tests for agrochemical formulations. Regul. Toxicol. Pharmacol. 2017, 89, 125–130. [Google Scholar] [CrossRef]
- Oesch, F.; Fabian, E.; Guth, K.; Landsiedel, R. Xenobiotic-metabolizing enzymes in the skin of rat, mouse, pig, guinea pig, man, and in human skin models. Arch. Toxicol. 2014, 88, 2135–2190. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, T.; Mehling, A.; Kolle, S.N.; Wruck, C.J.; Teubner, W.; Eltze, T.; Aumann, A.; Urbisch, D.; van Ravenzwaay, B.; Landsiedel, R. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol. In Vitro 2014, 28, 1482–1497. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, T.; Stein, N.; Aumann, A.; Remus, T.; Edwards, A.; Norman, K.G.; Ryan, C.; Bader, J.E.; Fehr, M.; Burleson, F.; et al. Intra- and inter-laboratory reproducibility and accuracy of the LuSens assay: A reporter gene-cell line to detect keratinocyte activation by skin sensitizers. Toxicol. In Vitro 2016, 32, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Teunis, M.A.T.; Spiekstra, S.W.; Smits, M.; Adriaens, E.; Eltze, T.; Galbiati, V.; Krul, C.; Landsiedel, R.; Pieters, R.; Reinders, J.; et al. International ring trial of the epidermal equivalent sensitizer potency assay: Reproducibility and predictive capacity. Altex-Alt. Anim. Exper. 2014, 31, 251–268. [Google Scholar] [CrossRef] [Green Version]
- Urbisch, D.; Becker, M.; Honarvar, N.; Kolle, S.N.; Mehling, A.; Teubner, W.; Wareing, B.; Landsiedel, R. Assessment of Pre- and Pro-haptens Using Nonanimal Test Methods for Skin Sensitization. Chem. Res. Toxicol. 2016, 29, 901–913. [Google Scholar] [CrossRef] [Green Version]
- Urbisch, D.; Honarvar, N.; Kolle, S.N.; Mehling, A.; Ramirez, T.; Teubner, W.; Landsiedel, R. Peptide reactivity associated with skin sensitization: The QSAR Toolbox and TIMES compared to the DPRA. Toxicol. In Vitro 2016, 34, 194–203. [Google Scholar] [CrossRef]
- Basketter, D.A.; Natsch, A.; Ellis, G.; Api, A.M.; Irizar, A.; Safford, B.; Ryan, C.; Kern, P. Interspecies assessment factors and skin sensitization risk assessment. Regul. Toxicol. Pharmacol. 2018, 97, 186–188. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R. Reporter cell lines for skin sensitization testing. Arch. Toxicol. 2015, 89, 1645–1668. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R. Nrf2 Activation as a Key Event Triggered by Skin Sensitisers: The Development of the Stable KeratinoSens Reporter Gene Assay. Atla-Altern. Lab. Anim. 2016, 44, 443–451. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R. Reaction Chemistry to Characterize the Molecular Initiating Event in Skin Sensitization: A Journey to Be Continued. Chem. Res. Toxicol. 2017, 30, 315–331. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R.; Haupt, T.; Ellis, G. Deriving a No Expected Sensitization Induction Level for Fragrance Ingredients Without Animal Testing: An Integrated Approach Applied to Specific Case Studies. Toxicol. Sci. 2018, 165, 170–185. [Google Scholar] [CrossRef]
- Nel, A.E.; Malloy, T.F. Policy reforms to update chemical safety testing. Science 2017, 355, 1016–1018. [Google Scholar] [CrossRef]
- EPA. Interim Science Policy: Use of Alternative Approaches for Skin Sensitization as a Replacement for Laboratory Animal Testing; (Draft for Public Comment); EPA: Washington, DC, USA, 2018.
- Daniel, A.B.; Strickland, J.; Allen, D.; Casati, S.; Zuang, V.; Barroso, J.; Whelan, M.; Régimbald-Krnel, M.J.; Kojima, H.; Nishikawa, A.; et al. International regulatory requirements for skin sensitization testing. Regul. Toxicol. Pharmacol. 2018, 95, 52–65. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Hill, E.; Curren, R.; Bishop, P. Bridging the Gap Between Regulatory Acceptance and Industry Use of Non-Animal Methods. Altex-Alt. Anim. Exper. 2016, 33, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Organisation for Economic Cooperation and Development (OECD). The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins; OECD Environment Directorate: Paris, France, 2014. [Google Scholar]
- Gerberick, G.F.; Vassallo, J.D.; Bailey, R.E.; Chaney, J.G.; Morrall, S.W.; Lepoittevin, J.P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Gerberick, G.F.; Vassallo, J.D.; Foertsch, L.M.; Price, B.B.; Chaney, J.G.; Lepoittevin, J.P. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree lmodel approach. Toxicol. Sci. 2007, 97, 417–427. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Test No. 442C: In Chemico Skin Sensitisation; OECD: Paris, France, 2019. [Google Scholar]
- Emter, R.; Ellis, G.; Natsch, A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol. Appl. Pharmacol. 2010, 245, 281–290. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Test No. 442D: In Vitro Skin Sensitisation; OECD: Paris, France, 2018. [Google Scholar]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT): I. Optimization of the h-CLAT protocol. Toxicol. In Vitro 2006, 20, 767–773. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Test No. 442E: In Vitro Skin Sensitisation; OECD: Paris, France, 2018. [Google Scholar]
- Rovida, C.; Alepee, N.; Api, A.M.; Basketter, D.A.; Bois, F.Y.; Caloni, F.; Corsini, E.; Daneshian, M.; Eskes, C.; Ezendam, J.; et al. Integrated Testing Strategies (ITS) for Safety Assessment. Altex-Alt. Anim. Exper. 2015, 32, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.; Zang, Q.D.; Kleinstreuer, N.; Paris, M.; Lehmann, D.M.; Choksi, N.; Matheson, J.; Jacobs, A.; Lowit, A.; Allen, D.; et al. Integrated decision strategies for skin sensitization hazard. J. Appl. Toxicol. 2016, 36, 1150–1162. [Google Scholar] [CrossRef]
- Strickland, J.; Zang, Q.D.; Paris, M.; Lehmann, D.M.; Allen, D.; Choksi, N.; Matheson, J.; Jacobs, A.; Casey, W.; Kleinstreuer, N. Multivariate models for prediction of human skin sensitization hazard. J. Appl. Toxicol. 2017, 37, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Zang, Q.D.; Paris, M.; Lehmann, D.M.; Bell, S.; Kleinstreuer, N.; Allen, D.; Matheson, J.; Jacobs, A.; Casey, W.; Strickland, J. Prediction of skin sensitization potency using machine learning approaches. J. Appl. Toxicol. 2017, 37, 792–805. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Hoffmann, S.; Alepee, N.; Allen, D.; Ashikaga, T.; Casey, W.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (II): An assessment of defined approaches. Crit. Rev. Toxicol. 2018, 48, 359–374. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). No. 497. Guideline on Defined Approaches for Skin Sensitisation; OECD: Paris, France, 2021. [Google Scholar]
- Natsch, A.; Gfeller, H.; Rothaupt, M.; Ellis, G. Utility and limitations of a peptide reactivity assay to predict fragrance allergens in vitro. Toxicol. In Vitro 2007, 21, 1220–1226. [Google Scholar] [CrossRef]
- Chipinda, I.; Mbiya, W.; Adigun, R.A.; Morakinyo, M.K.; Law, B.F.; Simoyi, R.H.; Siegel, P.D. Pyridoxylamine reactivity kinetics as an amine based nucleophile for screening electrophilic dermal sensitizers. Toxicology 2014, 315, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Chipinda, I.; Ajibola, R.O.; Morakinyo, M.K.; Ruwona, T.B.; Simoyi, R.H.; Siegel, P.D. Rapid and Simple Kinetics Screening Assay for Electrophilic Dermal Sensitizers Using Nitrobenzenethiol. Chem. Res. Toxicol. 2010, 23, 918–925. [Google Scholar] [CrossRef] [Green Version]
- NTP. Submissions of Test Methods for Evaluation. 2020. Available online: https://ntp.niehs.nih.gov/whatwestudy/niceatm/resources-for-test-method-developers/submissions/index.html (accessed on 31 March 2022).
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Gerberick, G.F.; Ryan, C.A.; Kern, P.S.; Schlatter, H.; Dearman, R.J.; Kimber, I.; Patlewicz, G.Y.; Basketter, D.A. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 2005, 16, 157–202. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Cooperation and Development (OECD). Supporting Document to the OECD Guideline 497 on Defined Approaches for Skin Sensitisation; OECD: Paris, France, 2021. [Google Scholar]
- Vanoirbeek, J.A.J.; De Vooght, V.; Synhaeve, N.; Nemery, B.; Hoet, P.H.M. Is Toluene Diamine a Sensitizer and is there Cross-Reactivity between Toluene Diamine and Toluene Diisocyanate? Toxicol. Sci. 2009, 109, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basketter, D.A.; Scholes, E.W.; Fielding, I.; Dearman, R.J.; Hilton, J.; Kimber, I. Dichloronitrobenzene: A reappraisal of its skin sensitization potential. Contact Dermat. 1996, 34, 55–58. [Google Scholar] [CrossRef]
- Kern, P.S.; Gerberick, G.F.; Ryan, C.A.; Kimber, I.; Aptula, A.; Basketter, D.A. Local Lymph Node Data for the Evaluation of Skin Sensitization Alternatives: A Second Compilation. Dermatitis 2010, 21, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Germolec, D.R.; Zhang, L.X.; Auttachoat, W.; Smith, M.J.; White, K.L., Jr. Contact sensitizing potential of pyrogallol and 5-amino-o-cresol in female BALB/c mice. Toxicology 2013, 314, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Auttachoat, W.; Germolec, D.R.; Smith, M.J.; White, K.L., Jr.; Guo, T.L. Contact sensitizing potential of annatto extract and its two primary color components, cis-bixin and norbixin, in female BALB/c mice. Food Chem. Toxicol. 2011, 49, 2638–2644. [Google Scholar] [CrossRef]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Dagli, M.L.; Fryer, A.D.; Greim, H.; Miyachi, Y.; Saurat, J.H.; Sipes, I.G. A toxicological and dermatological assessment of alkyl cyclic ketones when used as fragrance ingredients The RIFM Expert Panel. Food Chem. Toxicol. 2013, 62, S1–S44. [Google Scholar] [CrossRef]
- Stern, M.L.; Brown, T.A.; Brown, R.D.; Munson, A.E. Contact hypersensitivity response to isophorone diisocyanate in mice. Drug Chem. Toxicol. 1989, 12, 287–296. [Google Scholar] [CrossRef]
- Montelius, J.; Wahlkvist, H.; Boman, A.; Fernstrom, P.; Grabergs, L.; Wahlberg, J.E. Experience with the murine local lymph node assay: Inability to discriminate between allergens and irritants. Acta Derm. Venereol. 1994, 74, 22–27. [Google Scholar]
- Karrow, N.A.; Guo, T.L.; Leffel, E.K.; Zhang, L.X.; McCay, J.A.; Germolec, D.R.; White, K.L. Sodium metasilicate hypersensitivity in BALB/c mice. Am. J. Contact Dermat. 2002, 13, 133–139. [Google Scholar] [CrossRef]
- Ryan, C.A.; Gerberick, G.F.; Cruse, L.W.; Basketter, D.A.; Lea, L.; Blaikie, L.; Dearman, R.J.; Warbrick, E.V.; Kimber, I. Activity of human contact allergens in the murine local lymph node assay. Contact Dermat. 2000, 43, 95–102. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Van Och, F.M.M.; Jager, C.F.D.; Spiekstra, S.W.; Slob, W.; Vandebriel, R.J.; Van Loveren, H. Ranking of allergenic potency of rubber chemicals in a modified local lymph node assay. Toxicol. Sci. 2002, 66, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possolo, A.; Toman, B. Tutorial for Metrologists on the Probabilistic and Statistical Apparatus Underlying the GUM and Related Documents; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. Available online: www.itl.nist.gov/div898/possolo/TutorialWEBServer/TutorialMetrologists2011Nov09.xht (accessed on 31 March 2022).
- Gelman, A.; Carlin, J.; Stern, H.; Rubin, D. Bayesian Data Analysis, 2nd ed.; Chapman & Hall: London, UK, 2008. [Google Scholar]
- Toman, B.; Possolo, A. Laboratory effects models for interlaboratory comparisons. Accredit. Qual. Assur. 2009, 14, 553–563. [Google Scholar] [CrossRef]
- Lunn, D.; Spiegelhalter, D.; Thomas, A.; Best, N. The BUGS project: Evolution, critique and future directions. Stat. Med. 2009, 28, 3049–3067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Bauch, C.; Kolle, S.N.; Ramirez, T.; Eltze, T.; Fabian, E.; Mehling, A.; Teubner, W.; van Ravenzwaay, B.; Landsiedel, R. Putting the parts together: Combining in vitro methods to test for skin sensitizing potentials. Regul. Toxicol. Pharmacol. RTP 2012, 63, 489–504. [Google Scholar] [CrossRef]
- Nukada, Y.; Miyazawa, M.; Kazutoshi, S.; Sakaguchi, H.; Nishiyama, N. Data integration of non-animal tests for the development of a test battery to predict the skin sensitizing potential and potency of chemicals. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2013, 27, 609–618. [Google Scholar] [CrossRef]
- Cooper, J.A., 2nd; Saracci, R.; Cole, P. Describing the validity of carcinogen screening tests. Br. J. Cancer 1979, 39, 87–89. [Google Scholar] [CrossRef]
- Elliott, J.T.; Rosslein, M.; Song, N.W.; Toman, B.; Kinsner-Ovaskainen, A.; Maniratanachote, R.; Salit, M.L.; Petersen, E.J.; Sequeira, F.; Romsos, E.L.; et al. Toward Achieving Harmonization in a Nanocytotoxicity Assay Measurement Through an Interlaboratory Comparison Study. Altex-Alt. Anim. Exper. 2017, 34, 201–218. [Google Scholar] [CrossRef]
- Rösslein, M.; Elliott, J.T.; Salit, M.; Petersen, E.J.; Hirsch, C.; Krug, H.F.; Wick, P. Use of Cause-and-Effect Analysis to Design a High-Quality Nanocytotoxicology Assay. Chem. Res. Toxicol. 2015, 28, 21–30. [Google Scholar] [CrossRef]
- Hanna, S.K.; Cooksey, G.A.; Dong, S.; Nelson, B.C.; Mao, L.; Elliott, J.T.; Petersen, E.J. Feasibility of using a standardized Caenorhabditis elegans toxicity test to assess nanomaterial toxicity. Environ. Sci. Nano 2016, 3, 1080–1089. [Google Scholar] [CrossRef]
- Scanlan, L.D.; Lund, S.P.; Coskun, S.H.; Hanna, S.K.; Johnson, M.E.; Sims, C.M.; Brignoni, K.; Lapasset, P.; Petersen, E.J.; Elliott, J.T.; et al. Counting Caenorhabditis elegans: Protocol Optimization and Applications for Population Growth and Toxicity Studies in Liquid Medium. Sci. Rep. 2018, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Hirsch, C.; Elliott, J.T.; Krug, H.F.; Aengenheister, L.; Arif, A.T.; Bogni, A.; Kinsner-Ovaskainen, A.; May, S.; Walser, T.; et al. Cause-and-Effect Analysis as a Tool To Improve the Reproducibility of Nanobioassays: Four Case Studies. Chem. Res. Toxicol. 2020, 33, 1039–1054. [Google Scholar] [CrossRef]
- Leibrock, L.; Jungnickel, H.; Tentschert, J.; Katz, A.; Toman, B.; Petersen, E.J.; Bierkandt, F.S.; Singh, A.V.; Laux, P.; Luch, A. Parametric optimization of an air-liquid interface system for flow-through inhalation exposure to nanoparticles: Assessing dosimetry and intracellular uptake of CeO2 nanoparticles. Nanomaterials 2020, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Sharma, M.; Clippinger, A.J.; Gordon, J.; Katz, A.; Laux, P.; Leibrock, L.B.; Luch, A.; Matheson, J.; Stucki, A.O.; et al. Use of Cause-and-Effect Analysis to Optimize the Reliability of In Vitro Inhalation Toxicity Measurements Using an Air–Liquid Interface. Chem. Res. Toxicol. 2021, 34, 1370–1385. [Google Scholar] [CrossRef]
- Petersen, E.J.; Nguyen, A.D.; Brown, J.; Elliott, J.T.; Clippinger, A.J.; Gordon, J.; Kleinstreuer, N.; Roesslein, M. Characteristics to consider when selecting a positive control material for an in vitro assay. Altex Altern. Anim. Exp. 2021, 38, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.K.; Montoro Bustos, A.R.; Peterson, A.W.; Reipa, V.; Scanlan, L.D.; Hosbas Coskun, S.; Cho, T.J.; Johnson, M.E.; Hackley, V.A.; Nelson, B.C.; et al. Agglomeration of Escherichia coli with Positively Charged Nanoparticles Can Lead to Artifacts in a Standard Caenorhabditis elegans Toxicity Assay. Environ. Sci. Technol. 2018, 52, 5968–5978. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Petersen, E.J.; Marquis, B.J.; Atha, D.H.; Elliott, J.T.; Cleveland, D.; Watson, S.S.; Tseng, I.H.; Dillon, A.; Theodore, M.; et al. NIST gold nanoparticle reference materials do not induce oxidative DNA damage. Nanotoxicology 2013, 7, 21–29. [Google Scholar] [CrossRef]
- ISO 19007:2018; Nanotechnologies—In Vitro MTS Assay for Measuring the Cytotoxic Effect of Nanoparticles. International Organization for Standardization: Geneva, Switzerland, 2018.
- Patlewicz, G.; Casati, S.; Basketter, D.A.; Asturiol, D.; Roberts, D.W.; Lepoittevin, J.P.; Worth, A.P.; Aschberger, K. Can currently available non-animal methods detect pre and pro-haptens relevant for skin sensitization? Regul. Toxicol. Pharmacol. 2016, 82, 147–155. [Google Scholar] [CrossRef]
- Leontaridou, M.; Urbisch, D.; Kolle, S.N.; Ott, K.; Mulliner, D.S.; Gabbert, S.; Landsiedel, R. The Borderline Range of Toxicological Methods: Quantification and Implications for Evaluating Precision. Altex-Alt. Anim. Exper. 2017, 34, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Kolle, S.N.; Basketter, D.A.; Casati, S.; Stokes, W.S.; Strickland, J.; van Ravenzwaay, B.; Vohr, H.W.; Landsiedel, R. Performance standards and alternative assays: Practical insights from skin sensitization. Regul. Toxicol. Pharmacol. 2013, 65, 278–285. [Google Scholar] [CrossRef]
- Avonto, C.; Chittiboyina, A.G.; Rua, D.; Khan, I.A. A fluorescence high throughput screening method for the detection of reactive electrophiles as potential skin sensitizers. Toxicol. Appl. Pharmacol. 2015, 289, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Yamamoto, Y.; Tahara, H.; Kasahara, T.; Jimbo, Y.; Hioki, T. Development of a prediction method for skin sensitization using novel cysteine and lysine derivatives. J. Pharmacol. Toxicol. Methods 2014, 70, 94–105. [Google Scholar] [CrossRef]
- Wareing, B.; Kolle, S.N.; Birk, B.; Alépée, N.; Haupt, T.; Kathawala, R.; Kern, P.S.; Nardelli, L.; Raabe, H.; Rucki, M.; et al. The kinetic direct peptide reactivity assay (kdpra): Intra- and inter-laboratory reproducibility in a seven-laboratory ring trial. Altex-Alt. Anim. Exper. 2020, 37, 639–651. [Google Scholar] [CrossRef]
- Natsch, A.; Haupt, T.; Wareing, B.; Landsiedel, R.; Kolle, S.N. Predictivity of the kinetic direct peptide reactivity assay (kdpra) for sensitizer potency assessment and ghs subclassification. Altex-Alt. Anim. Exper. 2020, 37, 652–664. [Google Scholar]





| Chemical Name | CAS | DPRA Result | Reference a | In Vivo Result | Reference b | In Vivo Assay | LLNA EC3c | EASAd |
|---|---|---|---|---|---|---|---|---|
| 1,2-Propanediol | 57-55-6 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| 12-Bromo-1-dodecanol | 3344-77-2 | [37] | Sensitizer | [37] | LLNA | 6.9% | Nonbinder | |
| 1-Butanol | 71-36-3 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| 1-Butyl-1-methylpyrrolidinium chloride | 479500-35-1 | Nonbinder | ||||||
| 1-Butyl-3-methylimidazolium chloride | 79917-90-1 | Nonbinder | ||||||
| 1-Ethyl-3-methylimidazolium chloride | 65039-09-0 | Nonbinder | ||||||
| 1-Hydroxy-4-(p-toluidino) anthraquinone | 81-48-1 | Binder | ||||||
| 2,3-Butanedione | 431-03-8 | Binder | [37] | Sensitizer | [37,38] | LLNA | 11% | Binder |
| 2,4,5-Trichlorophenoxyacetic acid | 93-76-5 | Sensitizer | [39] | LLNA | 9.87% | Nonbinder | ||
| 2,4-Diaminotoluene | 95-80-7 | Sensitizer | [40] | LLNA | 19% | Binder | ||
| 2,4-Dichloronitrobenzene | 611-06-3 | Sensitizer | [41] | LLNA | Binder | |||
| 2-Amino-6-chloro-4-nitrophenol | 6358-09-4 | Sensitizer | [38,42] | LLNA | 2.2% | Binder | ||
| 2-Mercaptobenzothiazole | 149-30-4 | Binder | [37] | Sensitizer | [37] | LLNA | 1.7% | Binder |
| 2-Methoxy-4-nitroaniline | 97-52-9 | Nonsensitizer | [39] | GPMT | Binder | |||
| 2-Methyl-4H,3, 1-benzoxazin-4-one (Product 2040) | 525-76-8 | Sensitizer | [37] | LLNA | 0.7% | Inconclusive | ||
| 3,4-Dihydrocoumarin | 119-84-6 | Binder | [37] | Sensitizer | [37] | LLNA | 5.6% | Binder |
| 3-Iodo-2-propynyl butylcarbamate | 55406-53-6 | Binder | [37] | Sensitizer | [37] | LLNA | 0.9% | Binder |
| 4-Chloro-o-phenylenediamine | 95-83-0 | Binder | ||||||
| 4′-Hydroxychalcone | 2657-25-2 | Sensitizer | [42] | LLNA | 0.002% | Binder | ||
| 4-Methylcyclohexanemethanol | 34885-03-5 | Nonbinder | ||||||
| 4-Phenylenediamine | 106-50-3 | Binder | [37] | Sensitizer | [37] | LLNA | 0.16% | Binder |
| 5-Amino-o-cresol | 2835-95-2 | Binder | [37] | Sensitizer | [43] | LLNA | 3.4% | Binder |
| Ammonium thiosulfate | 7783-18-8 | Nonsensitizer | [39] | LLNA | Nonbinder | |||
| Aniline | 62-53-3 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 0.9% | Binder |
| Annatto | 1393-63-1 | Sensitizer | [44] | LLNA | 5% | Binder | ||
| Atrazine | 1912-24-9 | Sensitizer | [39] | LLNA | 31.3% to 41.4% | Nonbinder | ||
| Azithromycin | 83905-01-5 | Binder | ||||||
| Benzalkonium chloride | 8001-54-5 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| Benzethonium chloride | 121-54-0 | Nonbinder | ||||||
| Benzyl benzoate | 120-51-4 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 17% | Nonbinder |
| Benzyl bromide | 100-39-0 | Binder | [37] | Sensitizer | [37,38] | LLNA | 0.2% | Binder |
| Benzyl salicylate | 118-58-1 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 2.9% | Binder |
| Camphorquinone | 10373-78-1 | Sensitizer | [38] | LLNA | 10% | Binder | ||
| Chlorpyrifos | 2921-88-2 | Sensitizer | [39] | LLNA | 6.91% | Binder | ||
| Cinnamic aldehyde | 104-55-2 | Binder | [37] | Sensitizer | [37] | LLNA | 3.1% | Binder |
| Cinnamyl Alcohol | 104-54-1 | Binder | [37] | Sensitizer | [37] | LLNA | 21% | Binder |
| cis-Bixin | 6983-79-5 | Sensitizer | [44] | LLNA | 0.1% | Binder | ||
| Citral | 5392-40-5 | Binder | [37] | Sensitizer | [37] | LLNA | 4.6% to 13% | Binder |
| Clarithromycin | 81103-11-9 | Binder | ||||||
| D-glucose | 50-99-7 | Binder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| Dicyclohexylcarbodiimide | 538-75-0 | Binder | ||||||
| Diethyl maleate | 141-05-9 | Binder | [37] | Sensitizer | [37] | LLNA | 2.1% | Nonbinder |
| Dinitrochlorobenzene | 97-00-7 | Binder | [37] | Sensitizer | [37] | LLNA | 0.04% | Binder |
| Ethyl vanillin | 121-32-4 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| Ethylene thiourea | 96-45-7 | Nonbinder | ||||||
| Fluconazole | 86386-73-4 | Binder | ||||||
| Formaldehyde | 50-00-0 | Binder | [37] | Sensitizer | [38] | LLNA | 0.61% | Binder |
| Furil | 492-94-4 | Binder | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| Glutaraldehyde | 111-30-8 | Binder | [37] | Sensitizer | [37,38] | LLNA | 0.1% | Binder |
| Glycerol | 56-81-5 | Nonbinder | [37] | Nonsensitizer | [37,38] | LLNA | Nonbinder | |
| Glyoxal | 107-22-2 | Binder | [37] | Sensitizer | [37,38] | LLNA | 1.4% | Binder |
| Heptachlor (solution) | 76-44-8 | Nonbinder | ||||||
| Iso-E Super | 54464-57-2 | Sensitizer | [45] | LLNA | Binder | |||
| Isophorone diisocyanate | 4098-71-9 | Binder | [37] | Sensitizer | [37,46] | LLNA | 0.1% | Binder |
| Isopropanol | 67-63-0 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| Methyl pyruvate | 600-22-6 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 2.4% | Binder |
| Methyl salicylate | 119-36-8 | Nonbinder | [37] | Sensitizer | [38,47] | LLNA | Binder | |
| N,N-Diethyl-m-aminophenol | 91-68-9 | Sensitizer | [38] | LLNA | Binder | |||
| N,N-Dimethylformamide | 68-12-2 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| o-Benzyl-p-chlorophenol | 120-32-1 | Binder | ||||||
| o-Cresol | 95-48-7 | Binder | ||||||
| p,p′-Biphenol | 92-88-6 | Inconclusive | ||||||
| Palladium di(4-oxapent-2-en-2-oate) | 14024-61-4 | Binder | ||||||
| Penicillin | 61-33-6 | Binder | [37] | Sensitizer | [37] | LLNA | 30% | Nonbinder |
| Pentaerythritol triacrylate | 3524-68-3 | Binder | ||||||
| Perillaldehyde | 2111-75-3 | Binder | [37] | Sensitizer | [37] | LLNA | 8.1% | Binder |
| Phenylacetaldehyde | 122-78-1 | Binder | [37] | Sensitizer | [37] | LLNA | 3% to 4.7% | Binder |
| Potassium dicyanoaurate | 13967-50-5 | Nonbinder | ||||||
| Pyridine | 110-86-1 | Nonbinder | [37] | Sensitizer | [37,38] | LLNA | 71.2% | Nonbinder |
| Pyrogallol | 87-66-1 | Sensitizer | [44] | LLNA | 0.4% to 1.4% | Binder | ||
| R-Carvone | 6485-40-1 | Binder | [37] | Sensitizer | [37] | LLNA | 12.9% | Binder |
| Resorcinol | 108-46-3 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 5.92% | Nonbinder |
| Saccharin | 81-07-2 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Nonbinder | |
| Sodium dodecyl sulfate | 151-21-3 | Inconclusive | [37] | Sensitizer | [37] | LLNA | 14% | Nonbinder |
| Sodium metasilicate | 6834-92-0 | Nonsensitizer | [48] | LLNA | 2% to 6% | Inconclusive | ||
| Sodium octyl sulfate | 142-31-4 | Nonbinder | ||||||
| Squaric acid | 2892-51-5 | Binder | [37] | Sensitizer | [37,38,49] | LLNA | 4.3% | Binder |
| Streptomycin sulfate | 3810-74-0 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| Sulfanilamide | 63-74-1 | Nonbinder | [37] | Nonsensitizer | [37,38] | LLNA | Nonbinder | |
| Tetraethylthiuramdisulfide | 97-77-8 | Sensitizer | [38] | LLNA | 5.2% | Binder | ||
| Tetramethylthiuram disulfide | 137-26-8 | Binder | [37] | Sensitizer | [37] | LLNA | 3.1% | Binder |
| Tetramethylthiurammonosulfide | 97-74-5 | Sensitizer | [50] | LLNA | 5.4% | Binder | ||
| trans-2-Hexenal | 6728-26-3 | Binder | [37] | Sensitizer | [37] | LLNA | 5.5% | Binder |
| trans-p-Hydroxycinnamic acid | 501-98-4 | Binder | ||||||
| Triethanolamine | 102-71-6 | Nonsensitizer | [39] | GPMT | Binder | |||
| Trimethylolpropane triacrylate | 15625-89-5 | Sensitizer | [39] | LLNA | 0.01% to 0.13% | Inconclusive | ||
| Tri-n-octylphosphine oxide | 78-50-2 | Binder | ||||||
| Triphenyl phosphate | 115-86-6 | Nonsensitizer | [39] | GPMT | Nonbinder | |||
| Tween 80 | 9005-65-6 | Nonbinder | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| Vanillin | 121-33-5 | Inconclusive | [37] | Nonsensitizer | [37] | LLNA | Binder | |
| Zinc diethyldithiocarbamate | 14324-55-1 | Sensitizer | [50] | LLNA | 0.2% | Binder | ||
| α-Hexylcinnamaldehyde | 101-86-0 | Nonbinder | [37] | Sensitizer | [37] | LLNA | 12% | Binder |
| Bayesian α = 0.05 | Bayesian α = 0.01 | Bayesian α = 0.005 | Bayesian α = 0.001 | Frequentist t-Test α = 0.005 | 5 × Standard Deviation | DPRA | |
|---|---|---|---|---|---|---|---|
| GPMT and LLNA data | |||||||
| Agree | 70% (47/67) | 71% (46/65) | 69% (45/65) | 65% (45/69) | 73% (49/67) | 70% (46/66) | 77% (34/44) |
| False Positive | 53% (9/17) | 47% (7/15) | 47% (7/15) | 35% (6/17) | 47% (8/17) | 32% (6/19) | 15% (2/13) |
| False Negative | 22% (11/50) | 24% (12/50) | 26% (13/50) | 36% (18/50) | 20% (10/50) | 30% (14/47) | 26% (8/31) |
| Only LLNA data | |||||||
| Agree | 73% (47/64) | 73% (46/63) | 71% (45/63) | 67% (44/66) | 77% (49/64) | 71% (44/62) | 77% (34/44) |
| False Positive | 47% (7/15) | 43% (6/14) | 43% (6/14) | 33% (5/15) | 40% (6/15) | 31% (5/16) | 15% (2/13) |
| False Negative | 20% (10/49) | 22% (11/49) | 24% (12/49) | 35% (17/49) | 18% (9/49) | 28% (13/47) | 26% (8/31) |
| Performance Statistic | 2 out of 3 with EASA | 2 out of 3 with DPRA | KE 3/1 with EASA | KE 3/1 with DPRA |
|---|---|---|---|---|
| Accuracy | 79% (34/43) | 79% (33/42) | 83% (35/42) | 88% (37/42) |
| False Positive | 21% (3/14) | 8% (1/13) | 46% (6/13) | 8% (1/13) |
| False Negative | 21% (6/29) | 28% (8/29) | 3% (1/29) | 14% (4/29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, E.J.; Uhl, R.; Toman, B.; Elliott, J.T.; Strickland, J.; Truax, J.; Gordon, J. Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach. Toxics 2022, 10, 257. https://doi.org/10.3390/toxics10050257
Petersen EJ, Uhl R, Toman B, Elliott JT, Strickland J, Truax J, Gordon J. Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach. Toxics. 2022; 10(5):257. https://doi.org/10.3390/toxics10050257
Chicago/Turabian StylePetersen, Elijah J., Richard Uhl, Blaza Toman, John T. Elliott, Judy Strickland, James Truax, and John Gordon. 2022. "Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach" Toxics 10, no. 5: 257. https://doi.org/10.3390/toxics10050257
APA StylePetersen, E. J., Uhl, R., Toman, B., Elliott, J. T., Strickland, J., Truax, J., & Gordon, J. (2022). Development of a 96-Well Electrophilic Allergen Screening Assay for Skin Sensitization Using a Measurement Science Approach. Toxics, 10(5), 257. https://doi.org/10.3390/toxics10050257