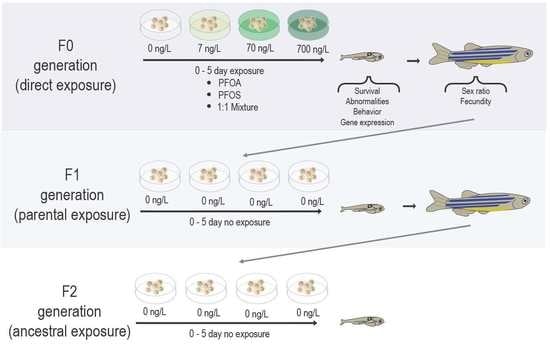
Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Husbandry of Adult Fish
2.2. PFAS Exposures
2.2.1. Spawning Procedure
2.2.2. Egg Cleaning
2.2.3. Exposure Protocol
2.3. Survival and Abnormality Screening
2.4. Behavioral Analysis
2.5. RNA-Seq and Pathway Analysis
2.6. Fecundity Assay
2.7. Sex Ratio
3. Results
3.1. F0 Generation
3.1.1. F0 Survival and Abnormalities
3.1.2. F0 Behavior
PFOA
PFOS
Mixture
3.1.3. F0 Transcriptomics
PFOA
PFOS
Mixture
3.1.4. F0 Fecundity
PFOA
PFOS
Mixture
3.1.5. F0 Adult Body Weight/Length
3.1.6. F0 Sex Ratio
3.2. F1 Generation
3.2.1. F1 Abnormalities and Survival
3.2.2. F1 Behavior
PFOA
PFOS
Mixture
3.2.3. F1 Transcriptomics and Pathway Analysis
PFOA
PFOS
Mixture
3.2.4. F1 Fecundity
PFOA
PFOS
Mixture
3.2.5. F1 Sex Ratio
PFOA
PFOS
Mixture
3.3. F2 Generation
3.3.1. F2 Abnormalities and Survival
3.3.2. F2 Behavior
PFOA
PFOS
Mixture
3.3.3. F2 Transcriptomics and Pathway Analysis
PFOA
PFOS
Mixture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Giesy, J.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Calafat, A.; Kato, K.; Hubbard, K.; Jia, T.; Botelho, J.; Wong, L. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int. 2019, 131, 105048. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Boone, J.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.; Kolpin, D.; Furlong, E.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef]
- Schultz, M.; Higgins, C.; Huset, C.; Luthy, R.; Barofsky, D.; Field, J. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 2006, 40, 7350–7357. [Google Scholar] [CrossRef] [Green Version]
- Gützkow, K.; Haug, L.; Thomsen, C.; Sabaredzovic, A.; Becher, G.; Brunborg, G. Placental transfer of perfluorinated com-pounds is selective—A Norwegian mother and child sub-cohort study. Int. J. Hyg. Environ. Health 2012, 215, 216–219. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Biomonitoring Data Tables for Environmental Chemicals. Available online: https://www.cdc.gov/exposurereport/data_tables.html (accessed on 6 June 2022).
- Baker, B.; Haimbaugh, A.; Sperone, F.; Johnson, D.; Baker, T. Persistent contaminants of emerging concern in a Great Lakes urban-dominant watershed. J. Great Lakes Res. 2022, 48, 171–182. [Google Scholar] [CrossRef]
- Ding, G.; Zhang, J.; Chen, Y.; Wang, L.; Wang, M.; Xiong, D.; Sun, Y. Combined effects of PFOS and PFOA on zebrafish (Danio rerio) embryos. Arch. Environ. Contam. Toxicol. 2013, 64, 668–675. [Google Scholar] [CrossRef]
- Menger, F.; Pohl, J.; Ahrens, L.; Carlsson, G.; Örn, S. Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 2020, 245, 125573. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thapar, I.; Brooks, B. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.; McCullough, S. Linking the epigenome with exposure effects and susceptibility: The epigenetic seed and soil model. Toxicol. Sci. 2016, 155, 302–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwmeester, M.; Ruiter, S.; Lommelaars, T.; Sippel, J.; Hodemaekers, H.; van den Brandhof, E.; Pennings, J.; Kamstra, J.; Jelinek, J.; Issa, J.; et al. Zebrafish embryos as a screen for DNA methylation modifications after compound exposure. Toxicol. Appl. Pharmacol. 2016, 291, 84–96. [Google Scholar] [CrossRef]
- Tian, J.; Xu, H.; Zhang, Y.; Shi, X.; Wang, W.; Gao, H.; Bi, Y. SAM targeting methylation by the methyl donor, a novel therapeutic strategy for antagonize PFOS transgenerational fertility toxicity. Ecotoxicol. Environ. Saf. 2019, 184, 109579. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, A. Memorandum: Directive to Prioritize Efforts to Reduce Animal Testing; Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- Hill, A.; Teraoka, H.; Heideman, W.; Peterson, R. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Baker, T.; King-Heiden, T.; Peterson, R.; Heideman, W. Dioxin induction of transgenerational inheritance of disease in zebrafish. Mol. Cell 2014, 398, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Fact Sheet: PFOA & PFOS Drinking Water Health Advisories. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf (accessed on 26 February 2022).
- Phillips, J.; Haimbaugh, A.; Akemann, C.; Shields, J.; Wu, C.; Meyer, D.; Baker, B.; Siddiqua, Z.; Pitts, D.; Baker, T. Devel-opmental phenotypic and transcriptomic effects of exposure to nanomolar levels of 4-nonylphenol, triclosan, and triclocarban in zebrafish (Danio rerio). Toxics 2022, 10, 53. [Google Scholar] [CrossRef]
- Ulhaq, M.; Carlsson, G.; Örn, S.; Norrgren, L. Comparison of developmental toxicity of seven perfluoroalkyl acids to zebrafish embryos. Environ. Toxicol. Pharmacol. 2013, 36, 423–426. [Google Scholar] [CrossRef]
- Sharpe, R.; Benskin, J.; Laarman, A.; MacLeod, S.; Martin, J.; Wong, C.; Goss, G. Perfluorooctane sulfonate toxicity, iso-mer-specific accumulation, and maternal transfer in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2010, 29, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.; Annunziato, K.; Bugel, S.; Cooper, K. PFOS, PFNA, And PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Du, Y.; Lam, P.; Wu, R.; Zhou, B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharmacol. 2008, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Orger, M.; de Polavieja, G. Zebrafish behavior: Opportunities and challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.; Gebhardt, M.; Stewart, A.; Cachat, J.; Brimmer, M.; Chawla, J.; Craddock, C.; Kyzar, E.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Jantzen, C.; Annunziato, K.; Cooper, K. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat. Toxicol. 2016, 180, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Ulhaq, M.; Örn, S.; Carlsson, G.; Morrison, D.; Norrgren, L. Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquat. Toxicol. 2013, 144–145, 332–340. [Google Scholar] [CrossRef]
- Gaballah, S.; Swank, A.; Sobus, J.; Howey, X.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ. Health Perspect. 2020, 128, 047005. [Google Scholar] [CrossRef] [Green Version]
- Khezri, A.; Fraser, T.; Nourizadeh-Lillabadi, R.; Kamstra, J.; Berg, V.; Zimmer, K.; Ropstad, E. A mixture of persistent organic pollutants and perfluorooctanesulfonic acid induces similar behavioural responses, but different gene expression profiles in zebrafish. Int. J. Mol. Sci. 2017, 18, 291. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Das, S.; La Du, J.; Corvi, M.; Bai, C.; Chen, Y.; Liu, X.; Zhu, G.; Tanguay, R.; Dong, Q.; et al. Chronic PFOS exposures induce life stage-specific behavioral deficits in adult zebrafish and produce malformation and behavioral deficits in F1 off-spring. Environ. Toxicol. Chem. 2012, 32, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Spulber, S.; Kilian, P.; Wan Ibrahim, W.; Onishchenko, N.; Ulhaq, M.; Norrgren, L.; Negri, S.; Di Tuccio, M.; Ceccatelli, S. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS ONE 2014, 9, e94227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.; Tanguay, R.; Dong, Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Chen, J.; Lin, K.; Chen, Y.; Hu, W.; Tanguay, R.; Huang, C.; Dong, Q. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ. Toxicol. Chem. 2011, 30, 2073–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, C.; Wolff, M.; Calafat, A.; Kato, K.; Engel, S. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: A pilot study. Reprod. Toxicol. 2012, 34, 312–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Ghisari, M.; Kjeldsen, L.; Wielsøe, M.; Nørgaard-Pedersen, B.; Mortensen, E.; Abdallah, M.; Bonefeld-Jørgensen, E. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: A case-control study. Mol. Autism 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.; Toor, F.; Annunziato, K.; Cooper, K. Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod. Toxicol. 2017, 69, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ZFIN Gene: Si:dkey-14d8.7.2022. Available online: https://zfin.org/ZDB-GENE-041210-143#summary (accessed on 20 April 2022).
- Marchak, A.; Grant, P.A.; Neilson, K.M.; Datta Majumdar, H.; Yaklichkin, S.; Johnson, D.; Moody, S.A. Wbp2nl has a developmental role in establishing neural and non-neural ectodermal fates. Dev. Biol. 2017, 429, 213–224. [Google Scholar] [CrossRef]
- Freour, T.; Barragan, M.; Ferrer-Vaquer, A.; Rodríguez, A.; Vassena, R. WBP2NL/PAWP mRNA and protein expression in sperm cells are not related to semen parameters, fertilization rate, or reproductive outcome. J. Assist. Reprod. Genet. 2017, 34, 803–810. [Google Scholar] [CrossRef]
- Dahlet, T.; Truss, M.; Frede, U.; Al Adhami, H.; Bardet, A.F.; Dumas, M.; Vallet, J.; Chicher, J.; Hammann, P.; Kottnik, S.; et al. E2F6 initiates stable epigenetic silencing of germline genes during embryonic development. Nat. Commun. 2021, 12, 3582. [Google Scholar] [CrossRef]
- Tissue Cell Type-WBP2NL-The Human Protein Atlas. Proteinatlas.org. 2022. Available online: https://www.proteinatlas.org/ENSG00000183066-WBP2NL/tissue+cell+type (accessed on 15 April 2022).
- Pierozan, P.; Jerneren, F.; Karlsson, O. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Arch. Toxicol. 2018, 92, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Stanifer, J.; Stapleton, H.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L. Perfluorinated chemicals as emerging envi-ronmental threats to kidney health. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, J.; Bautista, N.; Lucero, J.; Lund, A.; Xu, E.; Schlenk, D.; Burggren, W.; Roberts, A. Exposure to crude oil induces retinal apoptosis and impairs visual function in fish. Environ. Sci. Technol. 2020, 54, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, R.; Lantero, E.; Blanco-Kelly, F.; Avila-Fernandez, A.; Martin Merida, I.; del Pozo-Valero, M.; Perea-Romero, I.; Zurita, O.; Jiménez-Rolando, B.; Swafiri, S.; et al. RPE65-related retinal dystrophy: Mutational and phenotypic spectrum in 45 affected patients. Exp. Eye Res. 2021, 212, 108761. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.; O’Connor, J.; Dillon, E.; Duffy, D.; Kolch, W.; Cagney, G.; et al. A brain-derived neurotrophic factor mimetic is sufficient to restore cone photoreceptor visual function in an inherited blindness model. Sci. Rep. 2017, 7, 11320. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Vallanat, B.; Nelson, D.; Yeung, L.; Guruge, K.; Lam, P.; Lehman-McKeeman, L.; Corton, J. Evidence for the in-volvement of xenobiotic-responsive nuclear receptors in transcriptional effects upon perfluoroalkyl acid exposure in diverse species. Reprod. Toxicol. 2009, 27, 266–277. [Google Scholar] [CrossRef]
- Leeder, J.; Gaedigk, R.; Marcucci, K.; Gaedigk, A.; Vyhlidal, C.; Schindel, B.; Pearce, R. Variability of CYP3A7 expression in human fetal liver. J. Pharmacol. Exp. Ther. 2005, 314, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Raffi, F. Dual-specificity phosphatases in immunity and infection: An update. Int. J. Mol. Sci. 2019, 20, 2710. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. NTP monograph on immunotoxicity associated with exposure to per-fluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS). Natl. Toxicol. Prog. 2016, 22–80. [Google Scholar]
- Xin, Y.; Ren, X.; Wan, B.; Guo, L. Comparative in vitro and in vivo evaluation of the estrogenic effect of hexafluoropropylene oxide homologues. Environ. Sci. Technol. 2019, 53, 8371–8380. [Google Scholar] [CrossRef]
- Cardenas, A.; Gold, D.; Hauser, R.; Kleinman, K.; Hivert, M.; Calafat, A.; Ye, X.; Webster, T.; Horton, E.; Oken, E. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the diabetes prevention program trial. Environ. Health Perspect. 2017, 125, 107001. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Cardenas, A.; Hauser, R.; Gold, D.; Kleinman, K.; Hivert, M.; Fleisch, A.; Calafat, A.; Webster, T.; Horton, E.; et al. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults—longitudinal analysis of the diabetes pre-vention program outcomes study. Environ. Int. 2019, 129, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rajaobelina, K.; Praud, D.; Dow, C.; Antignac, J.; Kvaskoff, M.; Severi, G.; Bonnet, F.; Boutron-Ruault, M.; Fagherazzi, G. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: Findings from the E3N cohort study. Int. J. Hyg. Environ. Health 2018, 221, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.; Burks, D.; Towery, H.; Altamuro, S.; Flint, C.; White, M. Irs-2 coordinates igf-1 receptor-mediated β-cell de-velopment and peripheral insulin signalling. Nat. Genet. 1999, 23, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhai, G.; Gong, Y.; Su, J.; Peng, X.; Shang, G.; Han, D.; Jin, J.; Liu, H.; Du, Z.; et al. Different physiological roles of insulin receptors in mediating nutrient metabolism in zebrafish. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E38–E51. [Google Scholar] [CrossRef]
- Manchenkov, T.; Pasillas, M.; Haddad, G.; Imam, F. Novel genes critical for hypoxic preconditioning in zebrafish are reg-ulators of insulin and glucose metabolism. G3 Genes Genomes Genet. 2015, 5, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Raghupathy, R.; Zhang, X.; Alhasani, R.; Zhou, X.; Mullin, M.; Reilly, J.; Li, W.; Liu, M.; Shu, X. Abnormal photoreceptor outer segment development and early retinal degeneration inkif3a mutant zebrafish. Cell Biochem. Funct. 2016, 34, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Huang, H.; Hu, J.; Qin, Y.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere 2013, 91, 1099–1106. [Google Scholar] [CrossRef]
- Thisse, B.; Pflumio, S.; Fürthauer, M.; Loppin, B.; Heyer, V.; Degrave, A.; Woehl, R.; Lux, A.; Steffan, T.; Charbonnier, X.Q.; et al. Expression of the Zebrafish Genome during Embryogenesis (NIH R01 RR15402). ZFIN Direct Data Submission. 2001. Available online: https://www.scienceopen.com/document?vid=f98e6bdd-d74f-4d33-8aa4-4656a744c451 (accessed on 26 February 2022).
- Klarić, T.; Lardelli, M.; Key, B.; Koblar, S.; Lewis, M. Activity-dependent expression of neuronal pas domain-containing protein 4 (npas4a) in the developing zebrafish brain. Front. Neuroanat. 2014, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Han, H.; Kohwi-Shigematsu, T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat. Genet. 2003, 34, 42–51. [Google Scholar] [CrossRef]
- Yasui, D.; Miyano, M.; Cai, S.; Varga-Weisz, P.; Kohwi-Shigematsu, T. SATB1 targets chromatin remodelling to regulate genes over long distances. Nature 2002, 419, 641–645. [Google Scholar] [CrossRef]
- Martínez, R.; Navarro-Martín, L.; Luccarelli, C.; Codina, A.; Raldúa, D.; Barata, C.; Tauler, R.; Piña, B. Unravelling the mechanisms of PFOS toxicity by combining morphological and transcriptomic analyses in zebrafish embryos. Sci. Total Environ. 2019, 674, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, H.; Yu, Y.; Li, Y.; Naidu, R.; Liu, Y. Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: Trend and implications. Ecotoxicol. Environ. Saf. 2019, 173, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Hatch, E.; Webster, T. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010, 118, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, K.; Raaschou-Nielsen, O.; McLaughlin, J.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE 2013, 8, e56969. [Google Scholar] [CrossRef] [PubMed]
- Thisse, B.; Thisse, C. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission. 2004. Available online: https://zfin.org/ZDB-PUB-040907-1 (accessed on 26 February 2022).
- Lum, K.; Sundaram, R.; Barr, D.; Louis, T.; Buck Louis, G. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity. Epidemiology 2017, 28, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; McLaughlin, J.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitworth, K.; Haug, L.; Baird, D.; Becher, G.; Hoppin, J.; Skjaerven, R.; Thomsen, C.; Eggesbo, M.; Travlos, G.; Wilson, R.; et al. Perfluorinated compounds in relation to birth weight in the Norwegian mother and child cohort study. Am. J. Epidemiol. 2012, 175, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Velez, M.; Arbuckle, T.; Fraser, W. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 2015, 30, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, S.; Nielsen, F.; Andersson, A.; Hjollund, N.; Grandjean, P.; Andersen, H.; Jensen, T. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum. Reprod. 2012, 27, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, K.; Specht, I.; Lenters, V.; Bach, C.; Rylander, L.; Jönsson, B.; Lindh, C.; Giwercman, A.; Heederik, D.; Toft, G.; et al. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environ. Health 2014, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, A.; Pradhan, A.; Jass, J.; Olsson, P. Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci. Total Environ. 2020, 737, 139682. [Google Scholar] [CrossRef] [PubMed]
- Bangma, J.; Reiner, J.; Lowers, R.; Cantu, T.; Scott, J.; Korte, J.; Scheidt, D.; McDonough, C.; Tucker, J.; Back, B.; et al. Per-fluorinated alkyl acids and fecundity assessment in striped mullet (Mugil cephalus) at Merritt Island National Wildlife Refuge. Sci. Total Environ. 2018, 619–620, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marziali, L.; Rosignoli, F.; Valsecchi, S.; Polesello, S.; Stefani, F. Effects of perfluoralkyl substances on a multigenerational scale: A case study with Chironomus riparius (Diptera, Chironomidae). Environ. Toxicol. Chem. 2019, 38, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.; Salice, C.; Chanov, M.; Ayers, J.; Rewerts, J.; Field, J. Sensitivity and accumulation of perfluorooctanesulfonate and perfluorohexanesulfonic acid in fathead minnows (Pimephales promelas) exposed over critical life stages of reproduction and development. Environ. Toxicol. Chem. 2021, 40, 811–819. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Shin, Y.; Kim, J.; Ryu, T.; Ryu, J.; Lee, J.; Kim, P.; Choi, K.; Park, K. Multi-generational xenoestrogenic effects of perfluoroalkyl acids (pfaas) mixture on Oryzias latipes using a flow-through exposure system. Chemosphere 2017, 169, 212–223. [Google Scholar] [CrossRef]
- Nagabhushana, A.; Mishra, R. Finding clues to the riddle of sex determination in zebrafish. J. Biosci. 2016, 41, 145–155. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.; Hu, C.; Tsui, M.; Lam, P.; Zhou, B. Perfluorobutanesulfonate exposure skews sex ratio in fish and transgenerationally impairs reproduction. Environ. Sci. Technol. 2019, 53, 8389–8397. [Google Scholar] [CrossRef]
- Shi, G.; Guo, H.; Sheng, N.; Cui, Q.; Pan, Y.; Wang, J.; Guo, Y.; Dai, J. Two-generational reproductive toxicity assessment of 6:2 chlorinated polyfluorinated ether sulfonate (F-53B, a novel alternative to perfluorooctane sulfonate) in zebrafish. Environ. Pollut. 2018, 243, 1517–1527. [Google Scholar] [CrossRef]



| F0 Generation Endpoint | Concentration | PFOA | PFOS | Mixture |
| Survival | Ultra-low | |||
| Very low | ||||
| Low | ||||
| Morphological abnormalities | Ultra-low | |||
| Very low | ||||
| Low | ||||
| Swim distance (dark) | Ultra-low | −10.5% | +3.7% | |
| Very low | −10.2% | +3.6% | ||
| Low | −4.2% | +12.1% | ||
| Swim distance (light) | Ultra-low | −11.6% | +9% | |
| Very low | −18.8% | −8.16% | +9.7% | |
| Low | −5.4% | +16% | ||
| Differentially-expressed genes | Ultra-low | 1 | 6 | |
| Very low | 1 | 54 | ||
| Low | 14 | 2 | ||
| Fecundity | Ultra-low | +85% | ||
| Very low | +42.7% | |||
| Low | ||||
| Sex ratio (% males) | Ultra-low | |||
| Very low | ||||
| Low | ||||
| F1 Generation Endpoint | Concentration | PFOA | PFOS | Mixture |
| Survival | Ultra-low | |||
| Very low | ||||
| Low | +26.7 | |||
| Morphological abnormalities | Ultra-low | |||
| Very low | ||||
| Low | −7.4% | |||
| Swim distance (dark) | Ultra-low | +15.4% | −12.2% | |
| Very low | +4.6% | +10.2% | −9.9% | |
| Low | +9% | −12% | ||
| Swim distance (light) | Ultra-low | −15.5% | ||
| Very low | +9.6% | |||
| Low | +10.6% | −10.6% | ||
| Differentially-expressed genes | Ultra-low | 17 | 5 | 35 |
| Very low | 106 | 2 | 12 | |
| Low | 49 | 149 | 7 | |
| Fecundity | Ultra-low | |||
| Very low | ||||
| Low | −28.2% | |||
| Sex ratio (% males) | Ultra-low | +30.2% | ||
| Very low | +55.4% | +28.9% | ||
| Low | +57.1% | |||
| F2 Generation Endpoint | Concentration | PFOA | PFOS | Mixture |
| Survival | Ultra-low | |||
| Very low | ||||
| Low | ||||
| Morphological abnormalities | Ultra-low | |||
| Very low | ||||
| Low | ||||
| Swim distance (dark) | Ultra-low | −8.8% | −3.8% | |
| Very low | +9.4% | +11.2% | ||
| Low | −7.3% | |||
| Swim distance (light) | Ultra-low | −14.5% | ||
| Very low | −4.7% | +8.8% | ||
| Low | −14.8% | |||
| Differentially-expressed genes | Ultra-low | 112 | 484 | 69 |
| Very low | 106 | 23 | 1 | |
| Low | 302 | 7 | 9 |
| Gen. | Chemical | Conc. | Upregulated | Downregulated | Pathways |
|---|---|---|---|---|---|
| F0 | PFOA | Ultra-low | NA | NA | |
| Very low | NA | rpe65a | |||
| Low | ENSDARG00000075180, gabarapl2, capzb, npc2.1, dusp1 | atp6v0e1, trim36, phtf2, | |||
| PFOS | Ultra-low | irs2a | NA | ||
| Very low | irg1l, si:ch211-153b23.4, psma5, si:dkeyp-1h4.8, si:ch211-153b23.5 | kif3c, ENSDARG00000087345, map4k3b, prodha, ca4a | |||
| Low | NA | NA | NA | ||
| Mixture | Ultra-low | NA | fkbp9, si:ch211-251f6.6, hbbe1.3, ENSDARG00000092364, ENSDARG00000088687 | ||
| Very low | NA | NA | |||
| Low | slc6a19a.1, entpd8 | NA | |||
| F1 | PFOA | Ultra-low | dusp16, pycr1b, tmigd1, wbp2nl, serpina7 | zgc:136410, lgals1l1, pkhd1l1.2, pcnx3, si:ch211-125e6.5 | NA |
| Very low | dusp27, gadd45ba, lims2, asb2b, cuzd1.2 | c3a.2, c4b, mthfd1l, lgals1l1, si:ch211-125e6.5, | Xenobiotic metabolism, estrogen receptor signaling | ||
| Low | tmigd1, npas4a, dusp16, gadd45ba, dusp27 | trmt1, pitrm1, ercc6l, ifi44d, cdk16 | NA | ||
| PFOS | Ultra-low | satb1a, si:ch211-103n10.5, zgc:172051, spint1b, akap17a | NA | NA | |
| Very low | npas4a | slc43a2a | NA | ||
| Low | zmat5, ENSDARG00000082716, slc9a2, dusp19b, gadd45bb | mfsd14ba, ggt5b, ppp6r2b, dennd5a, nkx3.3 | Lipid metabolism, cell death | ||
| Mixture | Ultra-low | zgc:92590, smyhc2, amy2a, calcoco1b, si:dkey-14d8.7 | smtnl, fh, panx1a, trak2, g6pc1a.1 | NA | |
| Very low | cela1.3, si:dkey-14d8.7, amy2a, si:ch211-240l19.8, pla2g1b | panx1a | NA | ||
| Low | cpa4, zgc:92590, hsd11b2, pla2g1b, si:dkey-14d8.7 | ms4a17a.8, rlbp1b | NA | ||
| F2 | PFOA | Ultra-low | amy2al2, glg1a, slc17a6a, actl6a, ENSDARG00000096135 | ms4a17a.8, tfdp2, prss59.2, srsf5b, LOC100538179 | Mitochondrial membrane potential, organismal injury |
| Very low | amy2al2, crp2, LOC103910030, eef2k, pcnp | scn2b, cela1.3, cela1.5, tmem97, LOC101882496 | Cholesterol and other sterol synthesis | ||
| Low | amy2al2, LOC103910030, irg1l, eef2k, si:ch211-260e23.9 | lhx2b, smc1a, LOC110439320, rlbp1b, ms4a17a.8 | Immune cell function and trafficking, cell death, glucose homeostasis | ||
| PFOS | Ultra-low | cela1.5, haao, ENSDARG00000115830, atp9b, ENSDARG00000097916 | pgk1, si:ch211-260e23.9, crtac1a, cyp8b1, rrm2 | Steroid synthesis, bone mineral density, connective tissue | |
| Very low | cela1.5, lhx2b, cela1.3, mafb, zmp:0000001048 | b3gntl1, si:ch211-196h16.5, arpc5a, rbm4.1, bnip4 | NA | ||
| Low | ENSDARG00000115830, LOC100536187, pcdh1b, smdt1a | cfp, ddx47, LOC108179091 | NA | ||
| Mixture | Ultra-low | fzd6, gatm, bub3, fgfbp2b, il20ra | tcap, mmp9, bnip4, pfkfb3, si:dkey-85k7.7 | NA | |
| Very low | purab | hbae5, c4b, hbae1.3, cebpa | NA | ||
| Low | fgfbp2b, si:dkey-102c8.3 | si:ch211-281l24.3, anxa1c, si:ch211-240l19.8, calcoco1b, c4b | NA |
| Chemical | Gene Symbol | log2FC | p-Value | Function |
|---|---|---|---|---|
| PFOA | actl6a | 1.7 | 0.0018 | Chromatin modifying |
| foxa3 | 1.15 | 0.0067 | HAT recruitment | |
| glyr1 | 0.94 | 0.0085 | Nucleosome activity | |
| kdm3b | 0.99 | 0.0080 | Histone lysine demethylase | |
| mat1a | −1.6356 | 0.0035 | Methionine adenosyltransferase | |
| max | −1.24 | 0.0043 | HMT interaction | |
| sap30l | −1.18 | 0.0001 | HDAC subunit | |
| smc1a | −1.93 | 0.0000 | Chromatid tethering | |
| ybx1 | −1.33 (ultra-low); 1.06 (low) | <0.004 | DNA binding | |
| PFOS | chmp2a | −0.91 | 0.0079 | Chromatin modifying |
| h1-0 | 0.91 | 0.0028 | H1.0 linker histone | |
| hbp1 | 0.97 | 0.0017 | DNMT1 repressor | |
| hmg20a | 0.89 | 0.0085 | HMT recruitment | |
| hmgn2 | −1.06 | 0.0046 | Chromatin modifying | |
| hnrnpk | −1.19 | 0.0019 | ssDNA binding | |
| kdm1a | −0.87 | 0.0071 | Lysine demethylase 1A | |
| meaf6 | −1.4 | 0.0013 | HAT interactor | |
| prdm9 | −1.41 | 0.0053 | HMT recrutiment | |
| riox2 | 1.48 | 0.0004 | HDMT | |
| setd5 | 0.96 | 0.0020 | KMT2E paralog | |
| tox2 | 1.26 | 0.0001 | Chromatin modifying | |
| usf1 | −1.01 | 0.0071 | Chromatin modifying | |
| Mixture | h2bc1 | −0.95 | 0.0006 | H2B clustered histone 1 |
| Rank | Diseases or Functions Annotation | p-Value | Bias-Corrected z-Score | # Molecules |
|---|---|---|---|---|
| 1 | Organismal death | 3.59 × 10−3 | 1.714 | 22 |
| 3 | Morbidity or mortality | 1.81 × 10−3 | 1.429 | 23 |
| 10 | Quantity of cytokine | 3.53 × 10−3 | 0.834 | 5 |
| 11 | Infiltration by neutrophils | 1.30 × 10−3 | 0.793 | 5 |
| 12 | Cell movement of neutrophils | 3.56 × 10−3 | 0.751 | 6 |
| 18 | Necrosis | 6.16 × 10−3 | 0.603 | 23 |
| 24 | Chemotaxis of leukocytes | 9.40 × 10−4 | 0.307 | 7 |
| 27 | Quantity of myeloid cells | 1.41 × 10−3 | 0.301 | 9 |
| 41 | Cellular infiltration by phagocytes | 2.65 × 10−3 | −0.026 | 6 |
| 42 | Cellular infiltration by myeloid cells | 4.04 × 10−3 | −0.028 | 6 |
| 46 | Cellular infiltration by leukocytes | 1.09 × 10−3 | −0.144 | 8 |
| 57 | Accumulation of leukocytes | 5.69 × 10−3 | −0.402 | 5 |
| 87 | Inflammatory response | 5.20 × 10−3 | −1.872 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haimbaugh, A.; Wu, C.-C.; Akemann, C.; Meyer, D.N.; Connell, M.; Abdi, M.; Khalaf, A.; Johnson, D.; Baker, T.R. Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio). Toxics 2022, 10, 334. https://doi.org/10.3390/toxics10060334
Haimbaugh A, Wu C-C, Akemann C, Meyer DN, Connell M, Abdi M, Khalaf A, Johnson D, Baker TR. Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio). Toxics. 2022; 10(6):334. https://doi.org/10.3390/toxics10060334
Chicago/Turabian StyleHaimbaugh, Alex, Chia-Chen Wu, Camille Akemann, Danielle N. Meyer, Mackenzie Connell, Mohammad Abdi, Aicha Khalaf, Destiny Johnson, and Tracie R. Baker. 2022. "Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio)" Toxics 10, no. 6: 334. https://doi.org/10.3390/toxics10060334
APA StyleHaimbaugh, A., Wu, C.-C., Akemann, C., Meyer, D. N., Connell, M., Abdi, M., Khalaf, A., Johnson, D., & Baker, T. R. (2022). Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio). Toxics, 10(6), 334. https://doi.org/10.3390/toxics10060334