First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum
Abstract
:1. Introduction
2. Materials and Methods
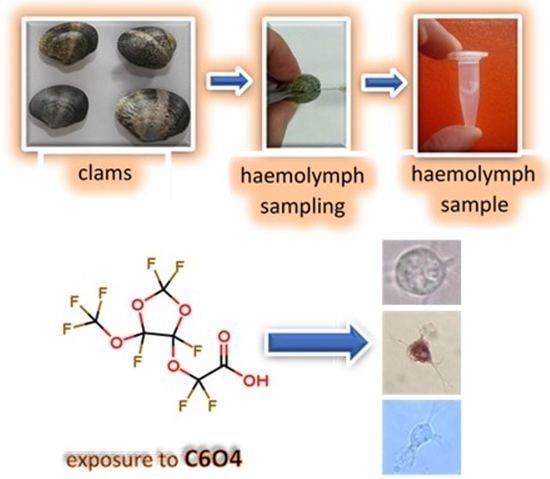
2.1. Clams, Haemolymph Collection, and Haemocyte Exposure
2.2. Haemocyte Viability Assay
2.3. Neutral Red Retention Assay
2.4. Haemocyte Morphology
2.5. Differential Cell Count (Pappenheim’s Panoptical Staining)
2.6. Hydrolytic Enzymes
2.7. Intracellular Superoxide Anion Detection
2.8. Micronuclei (MN) Assay
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sznajder-Katarzyńska, K.; Surma, M.; Cieślik, I. A review of perfluoroalkyl acids (PFAAs) in terms of sources, applications, human exposure, dietary intake, toxicity, legal regulation, and methods of determination. J. Chem. 2019, 2019, 2717528. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Exp. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [Green Version]
- UNEP. The Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 20 April 2021).
- Pistocchi, A.; Loos, R. A map of European emissions and concentrations of PFOS and PFOA. Environ. Sci. Technol. 2009, 43, 9237–9244. [Google Scholar] [CrossRef]
- Interstate Technology & Regulatory Council, USA. History and Use of Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://pfas1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_History_and_Use_April2020.pdf (accessed on 22 April 2021).
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, G.; Yao, J.; Sheng, N.; Cui, R.; Su, Z.; Guo, Y.; Dai, J. Perfluoropolyether carboxylic acids (novel alternatives to PFOA) impair zebrafish posterior swim bladder development via thyroid hormone disruption. Environ. Int. 2020, 134, 105317. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S. Presence of emerging per- and polyfluoroalkyl substances (pfass) in river and drinking water near a fluorochemical production plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef] [Green Version]
- Heydebreck, F.; Tang, J.; Xie, Z.; Ebinghaus, R. Alternative and legacy perfluoroalkyl substances: Differences between european and chinese river/estuary systems. Environ. Sci. Technol. 2015, 49, 8386–8395. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.; Sun, Y.; Guo, Y.; Dai, J. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ. Sci. Technol. 2018, 52, 7621–7629. [Google Scholar] [CrossRef] [PubMed]
- Joerss, H.; Apel, C.; Ebinghaus, R. Emerging per-and polyfluoroalkyl substances (PFASs) in surface water and sediment of the North and Baltic Seas. Sci. Total Environ. 2019, 686, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cheng, X.; Hua, X.; Jiang, B.; Tian, C.; Tang, J.; Li, Q.; Sun, H.; Lin, T.; Liao, Y.; et al. Emerging and legacy per-and polyfluoroalkyl substances in water, sediment, and air of the Bohai Sea and its surrounding rivers. Environ. Pollut. 2020, 263, 114391. [Google Scholar] [CrossRef]
- ECHA. Available online: https://echa.europa.eu/it/information-on-chemicals/registered-substances (accessed on 24 March 2021).
- Wang, D.; Goldenman, G.; Tugran, T.; McNeil, A.; Jones, M. Nordic Working Paper, Per- and Polyfluoroalkylether Substances: Identity, Production and Use. Nordic Council of Ministers, Copenhagen. 2020. Available online: https://www.norden.org/en/publication/and-polyfluoroalkylether-substances-identity-production-and-use-0 (accessed on 13 April 2021).
- Girardi, P.; Rosina, A.; Merler, E. La Concentrazione di Sostanze Perfluorurate nel Sangue dei Dipendenti ed ex Dipendenti Delle Ditte RIMAR e MITENI (Trissino, Vicenza). 2018. Available online: https://www.regione.veneto.it/web/sanita/informazione-e-comunicazione (accessed on 19 April 2021).
- ARPAV. Concentrazione di Sostanze Perfluoroalchiliche (PFAS) Nelle Acque Prelevate da ARPAV. Anni 2013–2020. Available online: https://www.arpa.veneto.it/dati-ambientali/open-data/idrosfera/concentrazione-di-sostanze-perfluoroalchiliche-pfas-nelle-acque-prelevate-da-arpav (accessed on 26 March 2021).
- Bernardini, I.; Matozzo, V.; Valsecchi, S.; Peruzza, L.; Dalla Rovere, G.; Polesello, S.; Iori, S.; Marin, M.G.; Fabrello, J.; Ciscato, M.; et al. The new PFAS C6O4 and its effects on marine invertebrates: First evidence of transcriptional and microbiota changes in the Manila clam Ruditapes philippinarum. Environ. Int. 2021, 152, 106484. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Matozzo, V.; Marin, M.G.; Ballarin, L. Haemocytes of the clam Tapes philippinarum (Adams & Reeve, 1850): Morphofunctional characterisation. Fish Shellfish Immunol. 2000, 10, 677–693. [Google Scholar] [PubMed]
- Donaghy, L.; Lambert, C.; Choi, K.; Soudant, P. Hemocytes of the carpet shell clam (Ruditapes decussatus) and the Manila clam (Ruditapes philippinarum): Current knowledge and future prospects. Aquaculture 2009, 297, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Ballarin, L.; Cima, F.; Sabbadin, A. Phagocytosis in the colonial ascidian Botryllus schlosseri. Dev. Comp. Immunol. 1994, 18, 467–481. [Google Scholar] [CrossRef]
- Lowe, D.M.; Fossato, V.U.; Depledge, M.H. Contaminant-induced lysosomal membrane damage in blood cells of mussels Mytilus galloprovincialis from the Venice lagoon: An in vitro study. Mar. Ecol. Prog. Ser. 1995, 129, 189–196. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakajima, Y.; Fishman, W.H. The cytologic demonstration of β-glucuronidase employing naphtol AS-BI glucuronide and hexazonium pararosanilin; a preliminary report. J. Histochem. Cytochem. 1964, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lojda, Z. Detection of acid phosphatase. In Proceedings of the 1st Conference of Czechoslovak Histochemical Committee, 28 September 1962. [Google Scholar]
- Song, Y.L.; Hsieh, Y.T. Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: Analysis of reactive oxygen species. Dev. Comp. Immunol. 1994, 18, 201–209. [Google Scholar] [CrossRef]
- Pavlica, M.; Klobučar, G.I.V.; Vetma, N.; Erben, R.; Papes, D. Detection of micronuclei in haemocytes of zebra mussel and ramshorn snail exposed to pentachlorophenol. Mutat. Res. 2000, 465, 145–150. [Google Scholar] [CrossRef]
- Kirsch-volders, M.; Toshio, S.; Marilyn, A.; Albertini, S.; David, E.; Michael, F.; Motoi, I.J.; Elisabeth, L.; Hannu, N.; Jordi, S.; et al. Report from the in vitro micronucleus assay working group. Environ. Mol. Mutagen. 2000, 2280, 167–172. [Google Scholar] [CrossRef]
- Olabarrieta, I.; L’Azou, B.; Yuric, S.; Cambar, J.; Cajaraville, M.P. In vitro effects of cadmium on two different animal cell models. Toxicol. Vitro 2001, 15, 511–517. [Google Scholar] [CrossRef]
- Liu, C.; Gin, K.Y. Immunotoxicity in green mussels under perfluoroalkyl substance (PFAS) exposure: Reversible response and response model development. Environ. Toxicol. Chem. 2018, 37, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, K.; Shi, X.; Wang, J.; Lam, P.K.; Wu, R.S.; Zhou, B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat. Toxicol. 2007, 82, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Croce, L.; Pignatti, P.; Ricci, G.; Gangemi, D.; Magri, F.; Imbriani, M.; Rotondi, M.; Chiovato, L. The new generation PFAS C6O4 does not produce adverse effects on thyroid cells in vitro. J. Endocrinol. Invest. 2020, 44, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Deblonde, T.; Diguio, N.; Hautemaniere, A.; Hartemann, P. Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: Cytotoxicity but no genotoxicity? Int. J. Hyg. Environ. Health 2011, 214, 493–499. [Google Scholar] [CrossRef]
- Lowe, D.M.; Moore, M.N.; Evans, B.M. Contaminant impact on interactions of molecular probes with lysosomes in living hepatocytes from dab Limanda limanda. Mar. Ecol. Prog. Ser. 1992, 91, 135–140. [Google Scholar] [CrossRef]
- Nobels, I.; Dardenne, F.; De Coen, W.; Blust, R. Application of a multiple endpoint bacterial reporter assay to evaluate toxicological relevant endpoints of perfluorinated compounds with different functional groups and varying chain length. Toxicol. Vitro 2010, 24, 1768–1774. [Google Scholar] [CrossRef]
- Hu, W.Y.; Jones, P.D.; DeCoen, W.; King, L.; Fraker, P.; Newsted, J.; Giesy, J.P. Alterations in cell membrane properties caused by perfluorinated compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 77–88. [Google Scholar] [CrossRef]
- Carballal, M.J.; Lòopez, C.; Azevedo, C.; Villalba, A. Enzymes involved in defense functions of hemocytes of mussel Mytilus galloprovincialis. J. Invertebr. Pathol. 1997, 70, 96–105. [Google Scholar] [CrossRef]
- Cima, F.; Marin, M.G.; Matozzo, V.; Da Ros, L.; Ballarin, L. Biomarkers for TBT immunotoxicity studies on the cultivated clam Tapes philippinarum (Adams and Reeve, 1850). Mar. Pollut. Bull. 1999, 39, 112–115. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS environmental pollution and antioxidant responses: An overview of the impact on human field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the ecological risks of per- and polyfluoroalkyl substances: Currentstate-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Cai, J.; Wang, S.; You, H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health 2017, 30, 213–229. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Men, B.; He, Y.; Xu, H.; Liu, M.; Wang, D. Effect of single-wall carbon nanotubes on bioconcentration and toxicity of perfluorooctane sulfonate in zebrafish (Danio rerio). Sci. Total Environ. 2017, 607–608, 509–518. [Google Scholar] [CrossRef]
- Liu, H.; Sheng, N.; Zhang, W.; Dai, J. Toxic effects of perfluorononanoic acid on the development of Zebrafish (Danio rerio) embryos. J. Environ. Sci. 2015, 32, 26–34. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Raaschou-Nielsen, O.; Sørensen, M.; Roursgaard, M.; Loft, S.; Møller, P. Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutat. Res. 2010, 700, 39–43. [Google Scholar] [CrossRef]
- Burgeot, T.; His, E.; Galgani, F. The micronucleus assay in Crassostrea gigas for the detection of seawater genotoxicity. Mutat. Res. 1995, 342, 125–140. [Google Scholar] [CrossRef]
- Bolognesi, C.; Landini, E.; Roggieri, P.; Fabbri, R.; Viarengo, A. Genotoxicity biomarkers in the assessment of heavy metal effects in mussels: Experimental studies. Environ. Mol. Mutagen. 1999, 33, 287–292. [Google Scholar] [CrossRef]
- Bolognesi, C.; Cirillo, S. Genotoxicity biomarkers in aquatic bioindicators. Curr. Zool. 2014, 60, 273–284. [Google Scholar] [CrossRef]
- Venier, P.; Maron, S.; Canova, S. Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo[a]pyrene. Mutat. Res. 1997, 390, 33–44. [Google Scholar] [CrossRef]
- Liu, C.; Chang, V.W.C.; Gin, K.Y.H.; Nguyen, V.T. Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). Sci. Total Environ. 2014, 487, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ayanda, I.O.; Yang, M.; Yu, Z.; Zha, J. Cytotoxic and genotoxic effects of perfluorododecanoic acid (PFDoA) in Japanese medaka. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 9. [Google Scholar] [CrossRef] [Green Version]
- Buhrke, T.; Kibellus, A.; Lampen, A. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicol. Lett. 2013, 218, 97–104. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabrello, J.; Targhetta, F.; Ciscato, M.; Asnicar, D.; Bernardini, I.; Milan, M.; Patarnello, T.; Marin, M.G.; Matozzo, V. First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum. Toxics 2021, 9, 191. https://doi.org/10.3390/toxics9080191
Fabrello J, Targhetta F, Ciscato M, Asnicar D, Bernardini I, Milan M, Patarnello T, Marin MG, Matozzo V. First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum. Toxics. 2021; 9(8):191. https://doi.org/10.3390/toxics9080191
Chicago/Turabian StyleFabrello, Jacopo, Francesca Targhetta, Maria Ciscato, Davide Asnicar, Ilaria Bernardini, Massimo Milan, Tomaso Patarnello, Maria Gabriella Marin, and Valerio Matozzo. 2021. "First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum" Toxics 9, no. 8: 191. https://doi.org/10.3390/toxics9080191
APA StyleFabrello, J., Targhetta, F., Ciscato, M., Asnicar, D., Bernardini, I., Milan, M., Patarnello, T., Marin, M. G., & Matozzo, V. (2021). First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum. Toxics, 9(8), 191. https://doi.org/10.3390/toxics9080191