Recently Developed Carbohydrate Based Gelators and Their Applications
Abstract
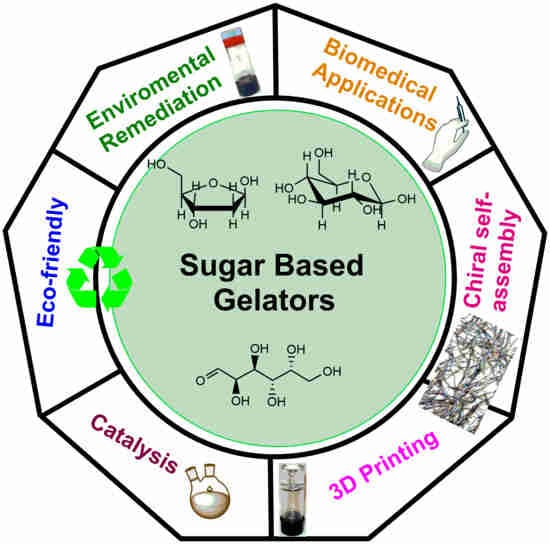
:1. Introduction
2. Structure and Gelation Properties of Monosaccharide Derivatives
2.1. D-Glucose Derivatives as LMWGs
2.2. D-Glucosamine and N-Acetyl D-Glucosamine Derivatives
2.2.1. Glucosamine Derivatives Functionalized at C-2 position from Methyl Glycosides
2.2.2. Functionalization at C-3 Positions
2.2.3. From Unprotected D-Glucosamine
2.3. D-Mannose, D-Arabinose, D-Galactose, Derivatives as LMOGs
2.4. Glyconamide Derivatives by Modification at the Anomeric Position
2.5. Alditol Derivatives from Reduced Sugar Alcohols
2.6. Glycosyl Triazole Derivatives as Molecular Gelators
2.6.1. C-1 Triazole Glycoside Derivatives
2.6.2. Carbohydrate Derivatives Containing Triazoles (not Anomeric Positions)
2.6.3. Glucosyl Triazole and Nucleoside Hybrid System
2.7. Disaccharide Derivatives and Glycoclusters
2.7.1. Disaccharide Derivatives
2.7.2. Branched Glycoclusters from Glycosyl Azides.
2.8. Nucleoside/Nucleotide-Based Gelators
2.8.1. 2-Deoxy Ribose Derivative as Gelators
2.8.2. From Ribose Derivatives
2.8.3. Nucleotides with Fluorescence Functions
3. Introduction to Stimuli-Responsive Gels
3.1. pH Responsive Gelators
3.2. Photosensitive Gelators
3.2.1. Photo-Isomerizable Gelators
3.2.2. Diacetylene Containing Photoreactive Gelators
3.3. Enzymatic Responsive Gelators and Their Biomedical Applications
4. Applications of Sugar-Based LMWGs
4.1. Environmental Remediation
4.2. Drug Delivery
4.3. Biomedical Applications: Antibacterial Agents and Wound Healing
4.4. Tissue Engineering
4.5. Shaping, Printing, Self-Healing and Thixotropy
5. Conclusions
Funding
Conflicts of Interest
References
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemitsu, H.; Hamachi, I. Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches. Accounts Chem. Res. 2017, 50, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Bibian, M.; Mangelschots, J.; Gardiner, J.; Waddington, L.; Acevedo, M.M.D.; De Geest, B.G.; Van Mele, B.; Madder, A.; Hoogenboom, R.; Ballet, S. Rational design of a hexapeptide hydrogelator for controlled-release drug delivery. J. Mater. Chem. B 2014, 3, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellama, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Wang, J.; Miao, X.; Fengzhao, Q.; Ren, C.; Yang, Z.; Wang, L. Using a mild hydrogelation process to confer stable hybrid hydrogels for enzyme immobilization. RSC Adv. 2013, 3, 16739–16746. [Google Scholar] [CrossRef]
- Kato, T.; Hirai, Y.; Nakaso, S.; Moriyama, M. Liquid-crystalline physical gels. Chem. Soc. Rev. 2007, 36, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Vibhute, A.M.; Sureshan, K.M. How Far Are We in Combating Marine Oil Spills by Using Phase-Selective Organogelators? ChemSusChem 2020, 13, 5343–5360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Co-ord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar] [CrossRef]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, G.; Zhang, Y. Organometallic Hydrogels. ChemNanoMat 2016, 2, 364–375. [Google Scholar] [CrossRef]
- Haring, M.; Diaz, D.D. Supramolecular metallogels with bulk self-healing properties prepared by in situ metal complexation. Chem. Commun. 2016, 52, 13068–13081. [Google Scholar] [CrossRef] [Green Version]
- Versluis, F.; Van Esch, J.H.; Eelkema, R. Synthetic Self-Assembled Materials in Biological Environments. Adv. Mater. 2016, 28, 4576–4592. [Google Scholar] [CrossRef]
- Tian, R.; Chen, J.; Niu, R. The development of low-molecular weight hydrogels for applications in cancer therapy. Nanoscale 2014, 6, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Escuder, B.; Rodriguez-Llansola, F.; Miravet, J.F. Supramolecular gels as active media for organic reactions and catalysis. New J. Chem. 2010, 34, 1044–1054. [Google Scholar] [CrossRef]
- Rodriguez-Llansola, F.; Escuder, B.; Miravet, J.F. Switchable performance of an L-proline-derived basic catalyst controlled by supramolecular gelation. J. Am. Chem. Soc. 2009, 131, 11478–11484. [Google Scholar] [CrossRef]
- Döring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels—Structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Zhang, K.; Angulo-Pachón, C.A.; Mendes, E.; Van Esch, J.H.; Escuder, B. Tandem reactions in self-sorted catalytic molecular hydrogels. Chem. Sci. 2016, 7, 5568–5572. [Google Scholar] [CrossRef] [Green Version]
- Basu, N.; Chakraborty, A.; Ghosh, R. Carbohydrate derived organogelators and the corresponding functional gels developed in recent time. Gels 2018, 4, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, R.; Liu, S.; Shigemitsu, H.; Nakamura, K.; Tanaka, W.; Ikeda, M.; Hamachi, I. Imaging-Based Study on Control Factors over Self-Sorting of Supramolecular Nanofibers Formed from Pep-tide- and Lipid-type Hydrogelators. Bioconjugate Chem. 2018, 29, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ouyang, G.-H.; Niu, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromol. Biosci. 2018, 19, e1800259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Feng, Z.; Xu, B. Supramolecular Assemblies of Peptides or Nucleopeptides for Gene Delivery. Theranostics 2019, 9, 3213–3222. [Google Scholar] [CrossRef]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar] [CrossRef]
- Soundarajan, K.; Periyasamy, R.; Mohan Das, T. Design and synthesis of sugar-benzohydrazides: Low molecular weight or-ganogelators. RSC Adv. 2016, 6, 81838–81846. [Google Scholar] [CrossRef]
- Wang, G.; Cheuk, S.; Williams, K.; Sharma, V.; Dakessian, L.; Thorton, Z. Synthesis and characterization of monosaccharide lipids as novel hydrogelators. Carbohydr. Res. 2006, 341, 705–716. [Google Scholar] [CrossRef]
- Nie, X.; Wang, G. Synthesis and Self-Assembling Properties of Diacetylene-Containing Glycolipids. J. Org. Chem. 2006, 71, 4734–4741. [Google Scholar] [CrossRef]
- Wang, G.; Yang, H.; Cheuk, S.; Coleman, S. Synthesis and self-assembly of 1-deoxyglucose derivatives as low molecular weight organogelators. Beilstein J. Org. Chem. 2011, 7, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Stevens, E.D.; Wang, G. Synthesis and structural analysis of a series of d-glucose derivatives as low molecular weight gelators. Carbohydr. Res. 2009, 344, 417–425. [Google Scholar] [CrossRef]
- Goyal, N.; Cheuk, S.; Wang, G. Synthesis and characterization of d-glucosamine-derived low molecular weight gelators. Tetrahedron 2010, 66, 5962–5971. [Google Scholar] [CrossRef]
- Wang, G.; Cheuk, S.; Yang, H.; Goyal, N.; Reddy, P.V.N.; Hopkinson, B. Synthesis and Characterization of Monosaccharide-Derived Carbamates as Low-Molecular-Weight Gelators. Langmuir 2009, 25, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Mangunuru, H.P.R.; Parikh, B.; Shrestha, S.; Wang, G. Synthesis and characterization of pH responsive D-glucosamine based molecular gelators. Beilstein J. Org. Chem. 2014, 10, 3111–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangunuru, H.P.R.; Yerabolu, J.R.; Wang, G. Synthesis and study of N-acetyl D-glucosamine triazole derivatives as effective low molecular weight gelators. Tetrahedron Lett. 2015, 56, 3361–3364. [Google Scholar] [CrossRef] [Green Version]
- Mangunuru, H.P.R.; Yang, H.; Wang, G. Synthesis of peptoid based small molecular gelators by a multiple component reaction. Chem. Commun. 2013, 49, 4489–4491. [Google Scholar] [CrossRef]
- Wang, G.; Goyal, N.; Mangunuru, H.P.R.; Yang, H.; Cheuk, S.; Reddy, P.V.N. Preparation and Self-Assembly Study of Amphiphilic and Bispolar Diacetylene-Containing Glycolipids. J. Org. Chem. 2015, 80, 733–743. [Google Scholar] [CrossRef]
- Mangunuru, H.P.R.; Yerabolu, J.R.; Liu, D.; Wang, G. Synthesis of a series of glucosyl triazole derivatives and their self-assembling properties. Tetrahedron Lett. 2015, 56, 82–85. [Google Scholar] [CrossRef]
- Wang, G.; Chen, A.; Mangunuru, H.P.R.; Yerabolu, J.R. Synthesis and characterization of amide linked triazolyl glycolipids as molecular hydrogelators and organo-gelators. RSC Adv. 2017, 7, 40887–40895. [Google Scholar] [CrossRef] [Green Version]
- Okafor, I.S.; Wang, G. Synthesis and gelation property of a series of disaccharide triazole derivatives. Carbohydr. Res. 2017, 451, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Adhikari, S.B.; Mays, K.; Wang, G. Synthesis and Study of Molecular Assemblies Formed by 4,6-O-(2-Phenylethylidene)-Functionalized d-Glucosamine Derivatives. Langmuir 2017, 33, 8076–8089. [Google Scholar] [CrossRef] [PubMed]
- Soundarajan, K.; Rajasekar, M.; Das, T.M. Self-assembly of sugar based glyco-lipids: Gelation studies of partially protected d-glucose derivatives. Mater. Sci. Eng. C 2018, 93, 776–781. [Google Scholar] [CrossRef]
- Ludwig, A.D.; Saint-Jalmes, A.; Mériadec, C.; Artzner, F.; Tasseau, O.; Berrée, F.; Lemiègre, L. Boron Effect on Sugar-Based Organogelators. Chem. A Eur. J. 2020, 26, 13927–13934. [Google Scholar] [CrossRef]
- Mahendar, C.; Dixit, M.K.; Kumar, Y.; Dubey, M. d-(+)-Glucose-triggered metallogel to metallogel transition. J. Mater. Chem. C 2020, 8, 11008–11012. [Google Scholar] [CrossRef]
- Lalitha, K.; Gayathri, K.; Prasad, Y.S.; Saritha, R.; Thamizhanban, A.; Maheswari, C.U.; Sridharan, V.; Nagarajan, S. Supramolecular Gel Formation Based on Glycolipids Derived from Renewable Resources. Gels 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Ono, F.; Ichimaru, K.; Hirata, O.; Shinkai, S.; Watanabe, H. Universal Glucose-based Low-molecular-weight Gelators for Both Organic and Aqueous Solvents. Chem. Lett. 2020, 49, 156–159. [Google Scholar] [CrossRef]
- Brinksma, J.; Feringa, B.L.; Kellogg, R.M.; Vreeker, R.; Van Esch, J. Rheology and Thermotropic Properties of Bis-Urea-Based Organogels in Various Primary Alcohols. Langmuir 2000, 16, 9249–9255. [Google Scholar] [CrossRef] [Green Version]
- Adarsh, N.; Kumar, D.K.; Dastidar, P. Composites of N,N′-bis-(pyridyl) urea-dicarboxylic acid as new hydrogelators—a crystal engineering approach. Tetrahedron 2007, 63, 7386–7396. [Google Scholar] [CrossRef]
- Suzuki, M.; Yumoto, M.; Shirai, H.; Hanabusa, K. A family of low-molecular-weight organogelators based on Nα,Nɛ-diacyl-l-lysine: Effect of alkyl chains on their organogelation behaviour. Tetrahedron 2008, 64, 10395–10400. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, Y.K.; Bhattacharya, S. Molecular mechanism of physical gelation of hydrocarbons by fatty acid amides of natural amino acids. Tetrahedron 2007, 63, 7334–7348. [Google Scholar] [CrossRef]
- Morris, J.; Kozlowski, P.; Wang, G. Synthesis and Characterization of Hybrid Glycolipids as Functional Organogelators and Hydrogelators. Langmuir 2019, 35, 14639–14650. [Google Scholar] [CrossRef]
- Chen, A.; Samankumara, L.P.; Garcia, C.; Bashaw, K.; Wang, G. Synthesis and characterization of 3-O-esters of N-acetyl-d-glucosamine derivatives as organogelators. New J. Chem. 2019, 43, 7950–7961. [Google Scholar] [CrossRef]
- Wang, D.; Chen, A.; Morris, J.; Wang, G. Stimuli-responsive gelators from carbamoyl sugar derivatives and their responses to metal ions and tetrabutylammonium salts. RSC Adv. 2020, 10, 40068–40083. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Zhou, Y.; Song, Q.; Zhang, R.; Lei, L.; Li, X. Glycosylation-enhanced biocompatibility of the supramolecular hydrogel of an anti-inflammatory drug for topical suppression of inflammation. Acta Biomater. 2018, 73, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef]
- Birchall, L.S.; Jayawarna, V.; Hughes, M.; Irvine, E.; Okorogheye, G.T.; Saudi, N.; De Santis, E.; Ulijn, R.V.; Roy, S.; Tuttle, T.; et al. Exploiting CH-π interactions in supramolecular hydrogels of aromatic carbohydrate amphiphiles. Chem. Sci. 2011, 2, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.; Abul-Haija, Y.M.; Soares da Costa, D.; Novoa-Carballal, R.; Reis, R.L.; Ulijn, R.V.; Pires, R.A.; Pashkuleva, I. Minimalistic supramolecular proteoglycan mimics by co-assembly of aromatic peptide and carbohydrate amphiphiles. Chem. Sci. 2019, 10, 2385–2390. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.; Pereira, P.; Reis, R.; Ulijn, R.; Lewis, J.S.; Pires, R.A.; Pashkuleva, I. Aromatic carbohydrate amphiphile disrupts cancer spheroids and prevents relapse. Nanoscale 2020, 12, 19088–19092. [Google Scholar] [CrossRef]
- Pal, K.B.; Mukhopadhyay, B. Carbohydrate-BasedSafe Fuel Gel with Significant Self–healing Property. ChemistrySelect 2017, 2, 967–974. [Google Scholar] [CrossRef]
- Pathak, N.P.; Rajkamal; Yadav, S. A gelator–starch blend for dry powder based instant solidification of crude oil at room temperature. Chem. Commun. 2020, 56, 2999–3002. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Arufe, S.; Martin, H.; Rooney, D.A.; Elmes, R.B.P.; Erxleben, A.; Moreira, R.; Velasco-Torrijos, T. Glycosyl squaramides, a new class of supramolecular gelators. Soft Matter 2020, 16, 7916–7926. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Muthuvijayan, V.; Prasad, E. In Vitro study of a glucose attached poly (aryl ether) dendron based gel as a drug carrier for a local anaesthetic. New J. Chem. 2017, 41, 7453–7462. [Google Scholar] [CrossRef]
- Chalard, A.; Vaysse, L.; Joseph, P.; Malaquin, L.; Souleille, S.; Lonetti, B.; Sol, J.-C.; Loubinoux, I.; Fitremann, J. Simple Synthetic Molecular Hydrogels from Self-Assembling Alkylgalactonamides as Scaffold for 3D Neuronal Cell Growth. ACS Appl. Mater. Interfaces 2018, 10, 17004–17017. [Google Scholar] [CrossRef]
- Chalard, A.; Joseph, P.; Souleille, S.; Lonetti, B.; Saffon-Merceron, N.; Loubinoux, I.; Vaysse, L.; Malaquin, L.; Fitremann, J. Wet spinning and radial self-assembly of a carbohydrate low molecular weight gelator into well organized hydrogel filaments. Nanoscale 2019, 11, 15043–15056. [Google Scholar] [CrossRef] [Green Version]
- Chalard, A.; Mauduit, M.; Souleille, S.; Joseph, P.; Malaquin, L.; Fitremann, J. 3D printing of a biocompatible low molecular weight supramolecular hydrogel by dimethylsulfoxide water solvent exchange. Addit. Manuf. 2020, 33, 101162. [Google Scholar] [CrossRef]
- Prasad, Y.S.; Manikandan, S.; Lalitha, K.; Sandeep, M.; Prasad, R.V.; Kumar, R.A.; Srinandan, C.; Maheswari, C.U.; Sridharan, V.; Nagarajan, S. Supramolecular gels of gluconamides derived from renewable resources: Antibacterial and anti-biofilm applications. Nano Sel. 2020, 1, 510–524. [Google Scholar] [CrossRef]
- Guan, X.; Fan, K.; Gao, T.; Ma, A.; Zhang, B.; Song, J. A novel multi-stimuli responsive gelator based on d -gluconic acetal and its potential applications. Chem. Commun. 2015, 52, 962–965. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Lin, P.; Zhang, N.; Han, X.; Zhang, B.; Song, J. Flexible and highly transparent two-component organogels with enhanced viscoelasticity for self-healing materials and room-temperature phase-selective gelation. Chem. Commun. 2016, 52, 13975–13978. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wang, X.; Yang, H.; Han, G.; Zhou, L.; Fang, S. One-step-synthesized d-gluconic acetal-based supramolecular organogelators with effective phase-selective gelation. RSC Adv. 2020, 10, 37080–37085. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Luo, H.; Zhang, B.; Wang, F.; Song, J. Porous amorphous powder form phase-selective organogelator for rapid recovery of leaked aromatics and spilled oils. J. Hazard. Mater. 2020, 384, 121460. [Google Scholar] [CrossRef]
- Lai, W.-C.; Huang, P.-H. Self-assembly behaviors of dibenzylidene sorbitol hybrid organogels with inorganic silica. Soft Matter 2017, 13, 3107–3115. [Google Scholar] [CrossRef]
- Dizon, G.C.; Atkinson, G.; Argent, S.P.; Santu, L.T.; Amabilino, D.B. Sustainable sorbitol-derived compounds for gelation of the full range of ethanol–water mixtures. Soft Matter 2020, 16, 4640–4654. [Google Scholar] [CrossRef]
- McNeice, P.; Zhao, Y.; Wang, J.; Donnelly, G.F.; Marr, P.C. Low molecular weight gelators (LMWGs) for ionic liquids: The role of hydrogen bonding and sterics in the formation of stable low molecular weight ionic liquid gels. Green Chem. 2017, 19, 4690–4697. [Google Scholar] [CrossRef] [Green Version]
- Raju, C.S.K.; Pramanik, B.; Ravishankar, R.; Rao, P.V.C.; Sriganesh, G. Xylitol based phase selective organogelators for potential oil spillage recovery. RSC Adv. 2017, 7, 37175–37180. [Google Scholar] [CrossRef] [Green Version]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Chen, A.; Okafor, I.S.; Garcia, C.; Wang, G. Synthesis and self-assembling properties of 4,6-O-benzylidene acetal protected D-glucose and D-glucosamine β-1,2,3-triazole derivatives. Carbohydr. Res. 2018, 461, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.P.; Sureshan, K.M. A Library of Multipurpose Supramolecular Supergelators: Fabrication of Structured Silica, Porous Plastics, and Fluorescent Gels. Chem. Asian J. 2017, 13, 187–193. [Google Scholar] [CrossRef]
- Rajkamal; Pathak, N.P.; Chatterjee, D.; Paula, A.; Yadav, S. Arabinose based gelators: Rheological characterization of the gels and phase selective organogelation of crude-oil. RSC Adv. 2016, 6, 92225–92234. [Google Scholar] [CrossRef]
- Narayana, C.; Kumari, P.; Tiwari, G.; Sagar, R. Triazole Linked N-Acetylglucosamine Based Gelators for Crude Oil Separation and Dye Removal. Langmuir 2019, 35, 16803–16812. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.A.; Baillet, J.; Benizri, S.; Latxague, L.; Barthélémy, P. Uracile based glycosyl-nucleoside-lipids as low molecular weight organogelators. New J. Chem. 2016, 40, 9903–9906. [Google Scholar] [CrossRef]
- Ramin, M.A.; Latxague, L.; Sindhu, K.R.; Chassande, O.; Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials 2017, 145, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Bansode, N.D.; Sindhu, K.R.; Morel, C.; Rémy, M.; Verget, J.; Boiziau, C.; Barthélémy, P. A disulfide based low molecular weight gel for the selective sustained release of biomolecules. Biomater. Sci. 2020, 8, 3186–3192. [Google Scholar] [CrossRef]
- Cano, M.E.; Di Chenna, P.H.; Lesur, D.; Wolosiuk, A.; Kovensky, J.; Uhrig, M.L. Chirality inversion, supramolecular hydrogelation and lectin binding of two thiolactose amphiphiles con-structed on a di-lauroyl-l-tartaric acid scaffold. New J. Chem. 2017, 41, 14754–14765. [Google Scholar] [CrossRef]
- Chen, A.; Wang, D.; Bietsch, J.; Wang, G. Synthesis and characterization of pentaerythritol derived glycoconjugates as supramolecular gelators. Org. Biomol. Chem. 2019, 17, 6043–6056. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, D.; Bietsch, J.; Chen, A.; Sharma, P. Synthesis of Dendritic Glycoclusters and Their Applications for Supramolecular Gelation and Catalysis. J. Org. Chem. 2020, 85, 16136–16156. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.M.; Davis, J.T. Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs. Chem. Soc. Rev. 2016, 45, 3188–3206. [Google Scholar] [CrossRef]
- Baillet, J.; Desvergnes, V.; Hamoud, A.; Latxague, L.; Barthélémy, P. Lipid and Nucleic Acid Chemistries: Combining the Best of Both Worlds to Construct Advanced Biomaterials. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Angelerou, M.G.F.; Frederix, P.W.J.M.; Wallace, M.; Yang, B.; Rodger, A.; Adams, D.J.; Marlow, M.; Zelzer, M. Supramolecular Nucleoside-Based Gel: Molecular Dynamics Simulation and Characterization of Its Nanoarchitecture and Self-Assembly Mechanism. Langmuir 2018, 34, 6912–6921. [Google Scholar] [CrossRef] [PubMed]
- Angelerou, M.G.F.; Yang, B.; Arnold, T.; Rawle, J.; Marlow, M.; Zelzer, M. Hydrophobicity of surface-immobilised molecules influences architectures formed via interfacial self-assembly of nucleoside-based gelators. Soft Matter 2018, 14, 9851–9855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Jiang, D.; Schäfer, A.H.; Seela, F. 8-Aza-2′-deoxyisoguanosine Forms Fluorescent Hydrogels whereas 8-Aza-2′-deoxyguanosine Assembles into Nucleoside Nanotubes. ChemPlusChem 2017, 82, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Alies, B.; Ouelhazi, M.A.; Patwa, A.N.; Verget, J.; Navailles, L.; Desvergnes, V.; Barthelemy, P. Cytidine- and guanosine-based nucleotide–lipids. Org. Biomol. Chem. 2018, 16, 4888–4894. [Google Scholar] [CrossRef]
- Nuthanakanti, A.; Srivatsan, S.G. Surface-Tuned and Metal-Ion-Responsive Supramolecular Gels Based on Nucleolipids. ACS Appl. Mater. Interfaces 2017, 9, 22864–22874. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, H.; Peng, Y.; Geng, L.; Zhu, L.; Cao, X.-Y.; Liu, C.-S.; Pang, H. A multifunctional self-healing G-PyB/KCl hydrogel: Smart conductive, rapid room-temperature phase-selective gelation, and ultrasensitive detection of alpha-fetoprotein. Chem. Commun. 2019, 55, 7922–7925. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, B.; Bishnoi, S.; Jain, S.; Nayak, D.; Sarma, T.K. Biocompatible Fe3+ and Ca2+ Dual Cross-Linked G-Quadruplex Hydrogels as Effective Drug Delivery System for pH-Responsive Sustained Zero-Order Release of Doxorubicin. ACS Appl. Bio Mater. 2019, 2, 3300–3311. [Google Scholar] [CrossRef]
- Nuthanakanti, A.; Walunj, M.B.; Torris, A.; Badiger, M.V.; Srivatsan, S.G. Self-assemblies of nucleolipid supramolecular synthons show unique self-sorting and cooperative assembling process. Nanoscale 2019, 11, 11956–11966. [Google Scholar] [CrossRef]
- Nuthanakanti, A. Cytidine and ribothymidine nucleolipids synthesis, organogelation, and selective anion and metal ion responsiveness. New J. Chem. 2019, 43, 13447–13456. [Google Scholar] [CrossRef]
- Nuthanakanti, A.; Srivatsan, S.G. Hierarchical self-assembly of switchable nucleolipid supramolecular gels based on environmentally-sensitive fluorescent nucleoside analogs. Nanoscale 2016, 8, 3607–3619. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, J.; Xu, S.; Zhang, F.; Sun, J.; Lu, R. Luminescent Organogels Generated from Nucleosides Functionalized with Carbazole: Synthesis and Probing for F-. Eur. J. Org. Chem. 2018, 2018, 1910–1915. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Gao, Y.; Kuang, Y.; Xu, B. Catalytic dephosphorylation of adenosine monophosphate (AMP) to form supramolecular nanofibers/hydrogels. Chem. Commun. 2011, 48, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M. Stimuli-responsive supramolecular systems guided by chemical reactions. Polym. J. 2018, 51, 371–380. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Cheetham, A.G.; Chakroun, R.W.; Ma, W.; Cui, H. Self-assembling prodrugs. Chem. Soc. Rev. 2017, 46, 6638–6663. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Priyanka, P. Environmentally benign pH-responsive cytidine-5′-monophosphate molecule-mediated akaganeite (5′-CMP-β-FeOOH) soft supramolecular hydrogels induced by the puckering of ribose sugar with efficient loading/release capabilities. New J. Chem. 2019, 43, 14997–15013. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Photoresponsive gelators. Chem. Commun. 2016, 52, 8196–8206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, M.J.; Tejedor, R.M.; Romero, P.; Fitremann, J.; Oriol, L. Maltose-based gelators having azobenzene as light-sensitive unit. RSC Adv. 2012, 2, 11419–11431. [Google Scholar] [CrossRef]
- Rajaganesh, R.; Gopal, A.; Das, T.M.; Ajayaghosh, A. Synthesis and Properties of Amphiphilic Photoresponsive Gelators for Aromatic Solvents. Org. Lett. 2012, 14, 748–751. [Google Scholar] [CrossRef]
- Oosumi, R.; Ikeda, M.; Ito, A.; Izumi, M.; Ochi, R. Structural diversification of bola-amphiphilic glycolipid-type supramolecular hydrogelators exhibiting colour changes along with the gel–sol transition. Soft Matter 2020, 16, 7274–7278. [Google Scholar] [CrossRef]
- Khayat, Z.; Zali-Boeini, H. Novel sugar-based azo dyes as multistimuli responsive supramolecular gelators and chemosensors. Dye. Pigment. 2018, 159, 337–344. [Google Scholar] [CrossRef]
- Lin, C.; Maisonneuve, S.; Métivier, R.; Xie, J. Photoswitchable Carbohydrate-Based Macrocyclic Azobenzene: Synthesis, Chiroptical Switching, and Multi-stimuli-Responsive Self-Assembly. Chem. Eur. J. 2017, 23, 14996–15001. [Google Scholar] [CrossRef]
- Baillet, J.; Gaubert, A.; Bassani, D.M.; Verget, J.; Latxague, L.; Barthélémy, P. Supramolecular gels derived from nucleoside based bolaamphiphiles as a light-sensitive soft material. Chem. Commun. 2020, 56, 3397–3400. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Raghu, S.; Mukherjee, S.; Sureshan, K.M. Organogel-assisted topochemical synthesis of multivalent glyco-polymer for high-affinity lectin binding. Chem. Commun. 2016, 52, 14089–14092. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, e1805798. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Baillet, J.; Gaubert, A.; Verget, J.; Latxague, L.; Barthélémy, P. β-Galactosidase instructed self-assembly of supramolecular blaamphiphiles hydrogelators. Soft Matter 2020, 16, 7648–7651. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cai, Y.; Zhong, X.; Zhang, L.; Zheng, D.; Gao, Z.; Pan, X.; Wang, F.; Chen, M.; Yang, Z. β-Galactosidase instructed supramolecular hydrogelation for selective identification and removal of senescent cells. Chem. Commun. 2019, 55, 7175–7178. [Google Scholar] [CrossRef]
- Zhou, J.; O’Keeffe, M.; Liao, G.; Zhao, F.; Terhorst, C.; Xu, B. Design and synthesis of nanofibers of self-assembled de novo glycoconjugates towards mucosal lining restoration and anti-inflammatory drug delivery. Tetrahedron 2016, 72, 6078–6083. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Du, X.; Chen, X.; Xu, B. Adaptive Multifunctional Supramolecular Assemblies of Glycopeptides Rapidly Enable Morphogenesis. Biochemistry 2018, 57, 4867–4879. [Google Scholar] [CrossRef]
- West, H.T.; Csizmar, C.M.; Wagner, C.R. Tunable Supramolecular Assemblies from Amphiphilic Nucleoside Phosphoramidate Nanofibers by Enzyme Activation. Biomacromolecules 2018, 19, 2650–2656. [Google Scholar] [CrossRef]
- Sekhar, K.P.C.; Swain, D.K.; Holey, S.A.; Bojja, S.; Nayak, R.R. Unsaturation and Polar Head Effect on Gelation, Bioactive Release, and Cr/Cu Removal Ability of Glycolipids. Langmuir 2020, 36, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Zhang, J.; Tian, X.; Li, X. A biocompatible supramolecular hydrogel with multivalent galactose ligands inhibiting Pseudomonas aeruginosa virulence and growth. RSC Adv. 2020, 10, 33642–33650. [Google Scholar] [CrossRef]
- Li, J.; Liang, S.; Yan, Y.; Tian, X.; Li, X. O-Mannosylation Affords a Glycopeptide Hydrogel with Inherent Antibacterial Activities against E. coli via Mul-tivalent Interactions between Lectins and Supramolecular Assemblies. Macromol. Biosci. 2019, 19, 1900124. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Goh, S.S.; Liow, S.S.; Xue, K.; Loh, X.J. Molecular gel sorbent materials for environmental remediation and wastewater treatment. J. Mater. Chem. A 2019, 7, 18759–18791. [Google Scholar] [CrossRef]
- Zhuan, C.; Li, Y.; Yuan, X.; Zhao, J.; Hou, X. A sorbitol-based phase-selective organogelator for crude oil spills treatment. J. Appl. Polym. Sci. 2019, 136, 47052. [Google Scholar] [CrossRef]
- Vibhute, A.M.; Muvvala, V.; Sureshan, K.M. A Sugar-Based Gelator for Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2016, 55, 7782–7785. [Google Scholar] [CrossRef]
- Soundarajan, K.; Das, T.M. Sugar-benzohydrazide based phase selective gelators for marine oil spill recovery and removal of dye from polluted water. Carbohydr. Res. 2019, 481, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Ma, Y.; Wang, X.; Yu, S.; Han, G.; Yin, Z.; Song, J. Sorbitol-based supramolecular organogelators with effective phase-selective gelation and significant self-healing property. Soft Mater. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Rizzo, C.; Andrews, J.L.; Steed, J.W.; D’Anna, F. Carbohydrate-supramolecular gels: Adsorbents for chromium(VI) removal from wastewater. J. Colloid Interface Sci. 2019, 548, 184–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, A.; Roy, S.G.; Haldar, U.; Mahapatra, R.D.; Harper, G.R.; Low, W.L.; De, P.; Hardy, J.G. Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels 2019, 5, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; He, X.; Zhong, M.; Hu, X.; Xiao, Y. A novel pH-responsive hydrogel based on natural polysaccharides for controlled release of protein drugs. RSC Adv. 2015, 5, 3157–3167. [Google Scholar] [CrossRef]
- Yoo, Y.; Yoon, S.-J.; Kim, S.Y.; Lee, D.-W.; Um, S.; Hyun, H.; Hong, S.O.; Yang, D.H. A local drug delivery system based on visible light-cured glycol chitosan and doxorubicin⋅hydrochloride for thyroid cancer treatment In Vitro and In Vivo. Drug Deliv. 2018, 25, 1664–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Sui, J.; Chen, Y.; Bian, S.; Cui, Y.; Zhou, C.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. Localized multidrug co-delivery by injectable self-crosslinking hydrogel for synergistic combinational chemotherapy. J. Mater. Chem. B 2017, 5, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Biswas, A.; Gavel, P.K.; Das, A.K. Engineered Dynamic Boronate Ester-Mediated Self-Healable Biocompatible G-Quadruplex Hydrogels for Sustained Release of Vitamins. Langmuir 2020, 36, 1574–1584. [Google Scholar] [CrossRef]
- Prasad, Y.S.; Miryala, S.; Lalitha, K.; Saritha, B.; Maheswari, C.U.; Sridharan, V.; Srinandan, C.S.; Nagarajan, S. An injectable self-healing anesthetic glycolipid-based oleogel with antibiofilm and diabetic wound skin repair properties. Sci. Rep. 2020, 10, 18017. [Google Scholar] [CrossRef]
- Prasad, Y.S.; Saritha, B.; Tamizhanban, A.; Lalitha, K.; Kabilan, S.; Maheswari, C.U.; Sridharan, V.; Nagarajan, S. Enzymatic synthesis and self-assembly of glycolipids: Robust self-healing and wound closure performance of assembled soft materials. RSC Adv. 2018, 8, 37136–37145. [Google Scholar] [CrossRef] [Green Version]
- Prasad, Y.S.; Miryala, S.; Lalitha, K.; Ranjitha, K.; Barbhaiwala, S.; Sridharan, V.; Maheswari, C.U.; Srinandan, C.S.; Nagarajan, S. Disassembly of Bacterial Biofilms by the Self-Assembled Glycolipids Derived from Renewable Resources. ACS Appl. Mater. Interfaces 2017, 9, 40047–40058. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Plank, T.N.; Zhu, T.; Yu, H.; Ge, Z.; Li, Q.; Li, L.; Davis, J.T.; Pei, H. Self-Assembly of Metallo-Nucleoside Hydrogels for Injectable Materials That Promote Wound Closure. ACS Appl. Mater. Interfaces 2019, 11, 19743–19750. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Chaudhuri, R.; Das, K.S.; Mondal, R.; Mandal, S.; Dash, J. Cytidine-Derived Hydrogels with Tunable Antibacterial Activities. ACS Appl. Bio. Mater. 2019, 2, 3171–3177. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, W.; Wu, F.; Wu, H.; He, B.; He, J. Low molecular weight gels induced differentiation of mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 3504–3508. [Google Scholar] [CrossRef] [PubMed]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.L.; Kim, Y.S.; Mikos, A.G. Biomacromolecules for Tissue Engineering: Emerging Biomimetic Strategies. Biomacromolecules 2019, 20, 2904–2912. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Ser. B Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Yan, Y.; Cheng, B.; Deng, L.; Shao, Z.; Sun, Z.; Li, X. Enzymatic Formation of an Injectable Hydrogel from a Glycopeptide as a Biomimetic Scaffold for Vascularization. ACS Appl. Mater. Interfaces 2018, 10, 6180–6189. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Fuentes-Caparrós, A.M.; Cross, E.R.; Cavalcanti, L.; Adams, D.J. Annealing Supramolecular Gels by a Reaction Relay. Chem. Mater. 2020, 32, 5264–5271. [Google Scholar] [CrossRef]
- Nolan, M.C.; Caparrós, A.M.F.; Dietrich, B.; Barrow, M.; Cross, E.R.; Bleuel, M.; King, S.M.; Adams, D.J. Optimising low molecular weight hydrogels for automated 3D printing. Soft Matter 2017, 13, 8426–8432. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Pekkanen, A.M.; Mondschein, R.J.; Williams, C.B.; Long, T.E. 3D Printing Polymers with Supramolecular Functionality for Biological Applications. Biomacromolecules 2017, 18, 2669–2687. [Google Scholar] [CrossRef]










































































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. https://doi.org/10.3390/gels7010024
Morris J, Bietsch J, Bashaw K, Wang G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels. 2021; 7(1):24. https://doi.org/10.3390/gels7010024
Chicago/Turabian StyleMorris, Joedian, Jonathan Bietsch, Kristen Bashaw, and Guijun Wang. 2021. "Recently Developed Carbohydrate Based Gelators and Their Applications" Gels 7, no. 1: 24. https://doi.org/10.3390/gels7010024
APA StyleMorris, J., Bietsch, J., Bashaw, K., & Wang, G. (2021). Recently Developed Carbohydrate Based Gelators and Their Applications. Gels, 7(1), 24. https://doi.org/10.3390/gels7010024