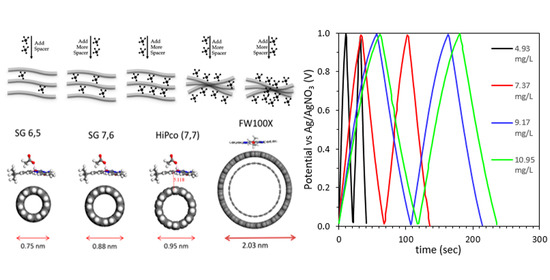
Characterization of Molecular Spacer-Functionalized Nanostructured Carbons for Electrical Energy Storage Supercapacitor Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Carbon Nanotube Dispersion and Spacer Solutions
2.2. Dispersion Stability Measurements
2.3. Adsorption Isotherm Measurements
2.4. Zeta Potential and Hydrodynamic Radius DLS Measurements
2.5. Molecular Spacer Characterization
2.5.1. 1H NMR (JEOL 500 MHz)
2.5.2. ATR FT-IR
2.5.3. UV-Vis-NIR Spectroscopy
2.5.4. Cyclic Voltammetry
2.6. EDL Capacitor Characterization
3. Results and Discussion
3.1. Dispersion Stability Measurements—Molecular Spacers on HiPco SWCNTs
3.2. Dispersion Stability Measurements: +2Ru2, +2Zn2, and Cu2FcOH on Various CNTs
3.3. Adsorption Isotherms
3.4. UV-Vis-NIR Spectroscopy
3.5. Raman Spectroscopy
3.6. Cyclic Voltammetry
3.7. Spectroelectrochemistry
3.8. EDL Capacitor Performance
3.9. Efficient Automated SWCNT@Carbon-Paper Working Electrodes
3.10. +2Ru2 Shows the Largest EDLC Enhancement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Hamzeh, M.; Mokhtari, H.; Karimi, H. Active Power Management of Multihybrid Fuel Cell/Supercapacitor Power Conversion System in a Medium Voltage Microgrid. IEEE Trans. Smart Grid 2012, 3, 1903–1910. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, L.; Zhang, J.J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.N.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alston, J.R.; Banks, D.J.; McNeill, C.X.; Mitchell, J.B.; Popov, L.D.; Shcherbakov, I.N.; Poler, J.C. Adsorption studies of divalent, dinuclear coordination complexes as molecular spacers on SWCNTs. Phys. Chem. Chem. Phys. 2015, 17, 29566–29573. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, B.; Maurer, L.; Lin, Z.; Lian, G.; Tuan, C.-C.; Moon, K.-S.; Wong, C.-P. Molecular engineering of aromatic amine spacers for high-performance graphene-based supercapacitors. Nano Energy 2016, 21, 276–294. [Google Scholar] [CrossRef]
- Song, B.; Sizemore, C.; Li, L.; Huang, X.; Lin, Z.; Moon, K.-s.; Wong, C.-P. Triethanolamine functionalized graphene-based composites for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 21789–21796. [Google Scholar] [CrossRef]
- Ameen, A.A.; Giordano, A.N.; Alston, J.R.; Forney, M.W.; Herring, N.P.; Kobayashi, S.; Ridlen, S.G.; Subaran, S.S.; Younts, T.J.; Poler, J.C. Aggregation kinetics of single-walled carbon nanotubes investigated using mechanically wrapped multinuclear complexes: Probing the tube-tube repulsive barrier. Phys. Chem. Chem. Phys. 2014, 16, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- Levchenkov, S.I.; Raspopova, E.A.; Morozov, A.N.; Popov, L.D.; Titova, Y.V.; Gorbunova, M.O.; Shcherbakov, I.N. 2,6-diformyl-4-tert-butylphenol bis-ferrocenoylhydrazone and binuclear copper(II) complexes on its basis. Russ. J. Gen. Chem. 2016, 86, 2075–2080. [Google Scholar] [CrossRef]
- Alston, J.R.; Kobayashi, S.; Younts, T.J.; Poler, J.C. Synthesis and characterization of rigid +2 and +3 heteroleptic dinuclear ruthenium(II) complexes. Polyhedron 2010, 29, 2696–2702. [Google Scholar] [CrossRef]
- Forney, M.W.; Poler, J.C. Significantly Enhanced Single-Walled Carbon Nanotube Dispersion Stability in Mixed Solvent Systems. J. Phys. Chem. C 2011, 115, 10531–10536. [Google Scholar] [CrossRef]
- Derjaguin, B.V. Theory of Stability of Colloids and Thin Films; Plenum Publishing Corporation: New York, NY, USA, 1989; p. 174. [Google Scholar]
- Derjaguin, B.V.; Landau, L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Acta Phys. Chim. Urss 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Forney, M.W.; Anderson, J.S.; Ameen, A.L.; Poler, J.C. Aggregation Kinetics of Single-Walled Carbon Nanotubes in Nonaqueous Solvents: Critical Coagulation Concentrations and Transient Dispersion Stability. J. Phys. Chem. C 2011, 115, 23267–23272. [Google Scholar] [CrossRef]
- Trivedi, R.; Deepthi, S.B.; Lingamallu, G.; Ramakrishna, K.V.S. Synthesis, Crystal Structure, Electronic Spectroscopy, Electrochemistry and Biological Studies of Ferrocene-Carbohydrate Conjugates. Eur. J. Inorg. Chem. 2012, 2012, 2267–2277. [Google Scholar] [CrossRef]
- Chaturvedi, H.; Giordano, A.N.; Kim, M.-J.; MacDonnell, F.M.; Subaran, S.S.; Poler, J.C. “Mechanically Docked” Metallodendrimers about Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 11254–11261. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; E, R. Smalley. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Leroux, F.; Beguin, F. A high-performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer. Adv. Mater. 2006, 18, 1877–1882. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]



















| Spacers | +2Ru2 | +2Cu2FcOH | +2Zn2 | Cu2FcOH |
|---|---|---|---|---|
| Dis. Stability X0 (µM) (HiPco) | 1.36 ± 0.02 | 5.9 ± 0.1 | 40.5 ± 0.4 | 110 ± 7 |
| CNTs | Diameter (nm) | Zeta Potential (mV) | X0 (µM) +2Ru2 | X0 (µM) +2Zn2 | X0 (µM) Cu2FcOH |
|---|---|---|---|---|---|
| SG 6,5 | 0.75 | −28 ± 6 | 1.37 ± 0.02 | 32.9 ± 0.4 | 77.4 ± 2.8 |
| SG 7,6 | 0.88 | −43 ± 11 | 22.7 ± 0.3 | 98 ± 20 | |
| HiPco | 0.95 | −51 ± 11 | 1.36 ± 0.02 | 40.5 ± 0.4 | 110 ± 7 |
| FW100X | 2.03 | −32 ± 6 | 65 ± 6 | 91 ± 8 | |
| MWCNT | 8.0 | 111 ± 3 |
| Sample | D (cm−1) | G−/G+ (cm−1) | G’ (cm−1) |
|---|---|---|---|
| Pristine HiPco film | 1290.6 | 1559.7/1587.0 | 2575.5 |
| +2Zn2 functionalized HiPco | 1288.2 | 1556.4/1583.4 | 2571.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuczek, J.; Bonfield, M.; Elathram, N.; R. Hixson, W.; Kongruengkit, T.; B. Mitchell, J.; Zelenka, N.; D. Popov, L.; Morozov, A.; N. Shcherbakov, I.; et al. Characterization of Molecular Spacer-Functionalized Nanostructured Carbons for Electrical Energy Storage Supercapacitor Materials. C 2020, 6, 66. https://doi.org/10.3390/c6040066
Zuczek J, Bonfield M, Elathram N, R. Hixson W, Kongruengkit T, B. Mitchell J, Zelenka N, D. Popov L, Morozov A, N. Shcherbakov I, et al. Characterization of Molecular Spacer-Functionalized Nanostructured Carbons for Electrical Energy Storage Supercapacitor Materials. C. 2020; 6(4):66. https://doi.org/10.3390/c6040066
Chicago/Turabian StyleZuczek, Justin, Matthew Bonfield, Nesreen Elathram, William R. Hixson, Terawit Kongruengkit, James B. Mitchell, Nickolas Zelenka, Leonid D. Popov, Andrey Morozov, Igor N. Shcherbakov, and et al. 2020. "Characterization of Molecular Spacer-Functionalized Nanostructured Carbons for Electrical Energy Storage Supercapacitor Materials" C 6, no. 4: 66. https://doi.org/10.3390/c6040066
APA StyleZuczek, J., Bonfield, M., Elathram, N., R. Hixson, W., Kongruengkit, T., B. Mitchell, J., Zelenka, N., D. Popov, L., Morozov, A., N. Shcherbakov, I., & C. Poler, J. (2020). Characterization of Molecular Spacer-Functionalized Nanostructured Carbons for Electrical Energy Storage Supercapacitor Materials. C, 6(4), 66. https://doi.org/10.3390/c6040066