Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pd-Modified KBs
2.3. Characterization Techniques
2.4. Enthalpy and Entropy Evaluation
3. Results and Discussion
3.1. Characterization of Samples
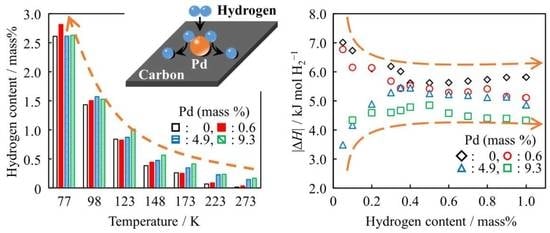
3.2. Hydrogen Adsorption
3.3. Enthalpy and Entropy Change
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Zou, Y.; Chu, H.; Li, B.; Zhang, K. Enhanced hydrogen storage/sensing of metal hydrides by nanomodification. Mater. Today Nano 2020, 9, 100071. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Sci. Nat. 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Mehrizi, M.Z.; Abdi, J.; Rezakazemi, M.; Salehi, E. A review on recent advances in hollow spheres for hydrogen storage. Int. J. Hydrog. Energy 2020, 45, 17583–17604. [Google Scholar] [CrossRef]
- Rather, S. Preparation, characterization and hydrogen storage studies of carbon nanotubes and their composites: A review. Int. J. Hydrog. Energy 2020, 45, 4653–4672. [Google Scholar] [CrossRef]
- Akasaka, H.; Takahata, T.; Toda, I.; Ono, H.; Ohshio, S.; Himeno, S.; Kokubu, T.; Saitoh, H. Hydrogen storage ability of porous carbon material fabricated from coffee bean wastes. Int. J. Hydrog. Energy 2011, 36, 580–585. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, L.; Fang, C.; Li, T.; Chen, J.; Yang, M.; Zhang, Q. Synthesis of porous carbon materials derived from laminaria japonica via simple carbonization and activation for supercapacitors. J. Mater. Res. Technol. 2020, 9, 3261–3271. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choi, S.W.; Kim, H.; Mun, S.; Lee, K.B. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem. Eng. J. 2020, 397, 125404. [Google Scholar] [CrossRef]
- Kim, B.-J.; Lee, Y.-S.; Park, S.-J. A study on the hydrogen storage capacity of Ni-plated porous carbon nanofibers. Int. J. Hydrog. Energy 2008, 33, 4112–4115. [Google Scholar] [CrossRef]
- Wu, H.; Wexler, D.; Ranjbartoreh, A.R.; Liu, H.; Wang, G. Chemical processing of double-walled carbon nanotubes for enhanced hydrogen storage. Int. J. Hydrog. Energy 2010, 35, 6345–6349. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lee, S.H.; Sim, K.S.; Kim, J.W. Synthesis and hydrogen storage of carbon nanofibers. Synth. Met. 2002, 126, 81–85. [Google Scholar] [CrossRef]
- Xiao, J.; Peng, R.; Cossement, D.; Bénard, P.; Chahine, R. Heat and mass transfer and fluid flow in cryo-adsorptive hydrogen storage system. Int. J. Hydrog. Energy 2013, 38, 10871–10879. [Google Scholar] [CrossRef]
- Hermosilla-Lara, G.; Momen, G.; Marty, P.H.; Le Neindre, B.; Hassouni, K. Hydrogen storage by adsorption on activated carbon: Investigation of the thermal effects during the charging process. Int. J. Hydrog. Energy 2007, 32, 1542–1553. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Dong, H.; Long, C.; Zheng, M.; Lei, B.; Zhang, H.; Liu, Y. Melaleuca bark based porous carbons for hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 11661–11667. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Influence of the pore size in multi-walled carbon nanotubes on the hydrogen storage behaviors. J. Solid State Chem. 2012, 194, 307–312. [Google Scholar] [CrossRef]
- Czakkel, O.; Nagy, B.; Dobos, G.; Fouquet, P.; Bahn, E.; László, K. Static and dynamic studies of hydrogen adsorption on nanoporous carbon gels. Int. J. Hydrog. Energy 2019, 44, 18169–18178. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, D.; Zhang, C.; Zhou, X.; Xin, H.; Liu, X.; Cai, M. Spillover enhanced hydrogen uptake of Pt/Pd doped corncob-derived activated carbon with ultra-high surface area at high pressure. Int. J. Hydrog. Energy 2014, 39, 13643–13649. [Google Scholar] [CrossRef]
- Minuto, F.D.; Policicchio, A.; Aloise, A.; Agostino, R.G. Liquid-like hydrogen in the micropores of commercial activated carbons. Int. J. Hydrog. Energy 2015, 40, 14562–14572. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X.; Gao, S. Adsorption equilibrium of hydrogen on graphene sheets and activated carbon. Cryogenics 2014, 61, 143–148. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chen, H.-M.; Chen, C.-H. Hydrogen adsorption on modified activated carbon. Int. J. Hydrog. Energy 2010, 35, 2777–2780. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Zlotea, C.; Izquierdo, M.T.; Chevalier-César, C.; Latroche, M.; Celzard, A. Activated carbons doped with Pd nanoparticles for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 5072–5080. [Google Scholar] [CrossRef]
- Kostoglou, N.; Tzitzios, V.; Kontos, A.G.; Giannakopoulos, K.; Tampaxis, C.; Papavasiliou, A.; Charalambopoulou, G.; Steriotis, T.; Li, Y.; Liao, K.; et al. Synthesis of nanoporous graphene oxide adsorbents by freeze-drying or microwave radiation: Characterization and hydrogen storage properties. Int. J. Hydrog. Energy 2015, 40, 6844–6852. [Google Scholar] [CrossRef]
- González-Navarro, M.F.; Giraldo, L.; Moreno-Piraján, J.C. Preparation and characterization of activated carbon for hydrogen storage from waste African oil-palm by microwave-induced LiOH basic activation. J. Anal. Appl. Pyrolysis 2014, 107, 82–86. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Li, S.; Wu, D.; Mao, Z. Measuring hydrogen storage capacity of carbon nanotubes by high-pressure microbalance. Int. J. Hydrog. Energy 2005, 30, 719–722. [Google Scholar] [CrossRef]
- Marella, M.; Tomaselli, M. Synthesis of carbon nanofibers and measurements of hydrogen storage. Carbon 2006, 44, 1404–1413. [Google Scholar] [CrossRef]
- Im, J.-E.; Oh, S.-L.; Choi, K.-H.; Wang, K.-K.; Jung, S.; Cho, W.; Oh, M.; Kim, Y.-R. Hydrogen uptake efficiency of mesoporous carbon nanofiber and its structural factors to determine the uptake efficiency. Surf. Coat. Technol. 2010, 205, S99–S103. [Google Scholar] [CrossRef]
- Knight, E.W.; Gillespie, A.K.; Prosniewski, M.J.; Stalla, D.; Dohnke, E.; Rash, T.A.; Pfeifer, P.; Wexler, C. Determination of the enthalpy of adsorption of hydrogen in activated carbon at room temperature. Int. J. Hydrog. Energy 2020, 45, 15541–15552. [Google Scholar] [CrossRef]
- Tellez-Juárez, M.C.; Fierro, V.; Zhao, W.; Fernández-Huerta, N.; Izquierdo, M.T.; Reguera, E.; Celzard, A. Hydrogen storage in activated carbons produced from coals of different ranks: Effect of oxygen content. Int. J. Hydrog. Energy 2014, 39, 4996–5002. [Google Scholar] [CrossRef]
- Kaneko, T.; Watanuki, Y.; Toyama, T.; Kojima, Y.; Nishimiya, N. Hydrogen sorption and desorption behaviors of metal-carbon composites prepared by alcohol CVD method. Int. J. Hydrog. Energy 2015, 40, 16323–16329. [Google Scholar] [CrossRef]
- Kaneko, T.; Watanuki, Y.; Toyama, T.; Kojima, Y.; Nishimiya, N. Characterization and hydrogen sorption behaviors of FeNiCr-carbon composites derived from Fe, Ni and Cr-containing polyacrylonitrile fibers prepared by electrospinning method. Int. J. Hydrog. Energy 2017, 42, 10014–10022. [Google Scholar] [CrossRef]
- Baca, M.; Cendrowski, K.; Banach, P.; Michalkiewicz, B.; Mijowska, E.; Kalenczuk, R.J.; Zielinska, B. Effect of Pd loading on hydrogen storage properties of disordered mesoporous hollow carbon spheres. Int. J. Hydrog. Energy 2017, 42, 30461–30469. [Google Scholar] [CrossRef]
- Stadie, N.P.; Purewal, J.J.; Ahn, C.C.; Fultz, B. Measurements of hydrogen spillover in platinum doped superactivated carbon. Langmuir 2010, 26, 15481–15485. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Gennett, T.; Atanassov, P.; Kurttepeli, M.; Bals, S.; Hurst, K.E.; Hirscher, M. Hydrogen adsorption properties of platinum decorated hierarchically structured templated carbons. Microporous Mesoporous Mater. 2013, 177, 66–74. [Google Scholar] [CrossRef]
- Klechikov, A.; Sun, J.; Hu, G.; Zheng, M.; Wågberg, T.; Talyzin, A.V. Graphene decorated with metal nanoparticles: Hydrogen sorption and related artefacts. Microporous Mesoporous Mater. 2017, 250, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, L. Experimental study on high-pressure adsorption of hydrogen on activated carbon. Sci. China Ser. B Chem. 1996, 39, 598–607. [Google Scholar] [CrossRef]
- Shen, L.; Ding, H.; Cao, Q.; Jia, W.; Wang, W.; Guo, Q. Fabrication of Ketjen black-high density polyethylene superhydrophobic conductive surfaces. Carbon 2012, 50, 4284–4290. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Contescu, C.I.; Benthem, K.; Li, S.; Bonifacio, C.S.; Pennycook, S.J.; Jena, P.; Gallego, N.C. Single Pd atoms in activated carbon fibers and their contribution to hydrogen storage. Carbon 2011, 49, 4050–4058. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wu, Z.-S.; Li, J.; Wu, E.-D.; Ren, W.-C.; Cheng, H.-M. Hydrogen adsorption behavior of graphene above critical temperature. Int. J. Hydrog. Energy 2009, 34, 2329–2332. [Google Scholar] [CrossRef]











| Sample | SBET | Vmicro 1 | Vmeso 2 |
|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (cm3 g−1) | |
| KB | 1925 | 0.290 | 2.09 |
| KB_0.6 | 1750 | 0.152 | 1.98 |
| KB_4.9 | 1686 | 0.203 | 1.81 |
| KB_9.3 | 1650 | 0.139 | 1.85 |
| Sample | Classification | Enthalpy | Temperature | Reference |
|---|---|---|---|---|
| (kJ mol H2−1) | (K) | |||
| AX-21 | Activated carbon | 6.4 1 | 77–298 | 35 |
| Litchi trunk based activated carbon | Activated carbon | 5.6–7.9 | 77–90 | 20 |
| SAC-02 | Activated carbon | 4.05–5.52 | 77.15–293.15 | 19 |
| Filtercarb GCC 8×30 | Activated carbon | 6.8 1–7.7 ± 0.4 2 | 77–318 | 18 |
| Filtercarb PHA | Activated carbon | 6.8 1–7.0 ± 0.4 2 | 77–318 | 18 |
| Nuchar SA-1500 | Activated carbon | 7.0 ± 1.3 1 | 77–318 | 18 |
| MWV-0260 3 | Activated carbon | 8.2 4 | 77–87 | 27 |
| KB | Carbon black | 5.7 ± 0.1–7.0 | 77–273 | The present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, T.; Toyama, T.; Kojima, Y.; Nishimiya, N. Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes. C 2022, 8, 16. https://doi.org/10.3390/c8010016
Kaneko T, Toyama T, Kojima Y, Nishimiya N. Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes. C. 2022; 8(1):16. https://doi.org/10.3390/c8010016
Chicago/Turabian StyleKaneko, Takehiro, Takeshi Toyama, Yoshiyuki Kojima, and Nobuyuki Nishimiya. 2022. "Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes" C 8, no. 1: 16. https://doi.org/10.3390/c8010016
APA StyleKaneko, T., Toyama, T., Kojima, Y., & Nishimiya, N. (2022). Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes. C, 8(1), 16. https://doi.org/10.3390/c8010016