Fabrication and Characterization of Flexible Three-Phase ZnO-Graphene-Epoxy Electro-Active Thin-Film Nanocomposites: Towards Applications in Wearable Biomedical Devices
Abstract
:1. Introduction
2. Methodology and Procedure
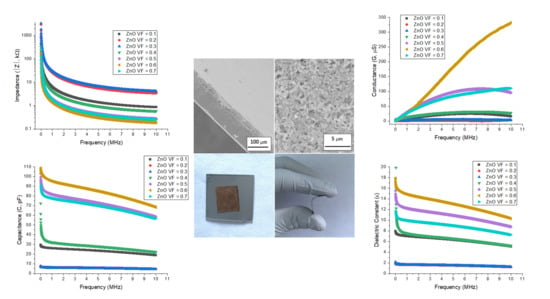
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.; Harris, J.; Shaffer, J.; Daniel, A.; Chowdhury, S.; Ali, A.; Banerjee, S.; Ahmed, S. Bismuth perovskite as a viable alternative to Pb perovskite solar cells: Device simulations to delineate critical efficiency dynamics. J. Mater. Sci. Mater. Electron. 2019, 30, 9438–9443. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Effect of grain size on the domain structures and electromechanical responses of ferroelectric polycrystal. Smart Mater. Struct. 2016, 26, 015013. [Google Scholar] [CrossRef]
- Mokrý, P.; Psota, P.; Steiger, K.; Václavík, J.; Doleček, R.; Vápenka, D.; Lédl, V. Ferroelectric domain pattern in barium titanate single crystals studied by means of digital holographic microscopy. J. Phys. D Appl. Phys. 2016, 49, 255307. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Xiao, Z.; Yang, B.; Huang, J. Arising applications of ferroelectric materials in photovoltaic devices. J. Mater. Chem. A 2014, 2, 6027–6041. [Google Scholar] [CrossRef] [Green Version]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Turkevych, I.; Kazaoui, S.; Ito, E.; Urano, T.; Yamada, K.; Tomiyasu, H.; Yamagishi, H.; Kondo, M.; Aramaki, S. Photovoltaic Rudorffites: Lead-Free Silver Bismuth Halides Alternative to Hybrid Lead Halide Perovskites. ChemSusChem 2017, 10, 3754–3759. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Ho, J.C.; Ko, H.; Gillies, A.G.; Leu, P.W.; Fearing, R.S.; Javey, A. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010, 9, 821–826. [Google Scholar] [CrossRef]
- Pang, C.; Lee, C.; Suh, K.Y. Recent advances in flexible sensors for wearable and implantable devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Fan, Z.; Ho, J.C.; Jacobson, Z.A.; Razavi, H.; Javey, A. Large-scale, heterogeneous integration of nanowire arrays for image sensor circuitry. Natl. Acad. Sci. 2008, 105, 11066–11070. [Google Scholar] [CrossRef] [Green Version]
- Abiri, H.; Abdolahad, M.; Gharooni, M.; Ali Hosseini, S.; Janmaleki, M.; Azimi, S.; Hosseini, M.; Mohajerzadeh, S. Monitoring the spreading stage of lung cells by silicon nanowire electrical cell impedance sensor for cancer detection purposes. Biosens. Bioelectron. 2015, 68, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Ma, X.; Li, N.; Yang, X. Label-free and sensitive thrombin sensing on a molecularly grafted aptamer on graphene. Chem. Commun. 2012, 48, 738–740. [Google Scholar] [CrossRef]
- Kurkina, T.; Vlandas, A.; Ahmad, A.; Kern, K.; Balasubramanian, K. Label-Free Detection of Few Copies of DNA with Carbon Nanotube Impedance Biosensors. Angew. Chem. Int. Ed. Engl. 2011, 50, 3710–3714. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, C.; Arcay, E.; del Valle, M. Label-free impedimetric aptasensor based on epoxy-graphite electrode for the recognition of cytochrome c. Sens. Actuators B Chem. 2014, 191, 860–865. [Google Scholar] [CrossRef]
- Ahmad, M.; Pan, C.; Luo, Z.; Zhu, J. A Single ZnO Nanofiber-Based Highly Sensitive Amperometric Glucose Biosensor. J. Phys. Chem. C 2010, 114, 9308–9313. [Google Scholar] [CrossRef]
- SoYoon, S.; Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Novel Cu/CuO/ZnO hybrid hierarchical nanostructures for non-enzymatic glucose sensor application. J. Electroanal. Chem. 2014, 717, 90–95. [Google Scholar] [CrossRef]
- Wu, W.; Miao, F.; Tao, B.; Zang, Y.; Zhu, L.; Shi, C.; Chu, P.K. Hybrid ZnO–graphene electrode with palladium nanoparticles on Ni foam and application to self-powered nonenzymatic glucose sensing. RSC Adv. 2019, 9, 12134–12145. [Google Scholar] [CrossRef] [Green Version]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. A facile synthesis of ZnO/Manganese hexacyanoferrate nanocomposite modified electrode for the electrocatalytic sensing of riboflavin. J. Phys. Chem. Solids 2018, 121, 350–359. [Google Scholar] [CrossRef]
- Felix, S.; Kollu, P.; Grace, A.N. Electrochemical performance of Ag–CuO nanocomposites towards glucose sensing. Mater. Res. Innov. 2019, 23, 27–32. [Google Scholar] [CrossRef]
- Wu, J.; Yin, F. Easy fabrication of a sensitive non-enzymatic glucose sensor based on electrospinning CuO-ZnO nanocomposites. Integr. Ferroelectr. 2013, 147, 47–58. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Janssen, R.A. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Ellmer, K. Resistivity of polycrystalline zinc oxide films: Current status and physical limit. J. Phys. D Appl. Phys. 2001, 34, 3097–3108. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc Oxide Nanostructure-Modified Textile and Its Application to Biosensing, Photocatalysis, and as Antibacterial Material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Palanchoke, U.; Kurz, H.; Noriega, R.; Arabi, S.; Jovanov, V.; Magnus, P.; Aftab, H.; Salleo, A.; Stiebig, H.; Knipp, D. Tuning the plasmonic absorption of metal reflectors by zinc oxide nano particles: Application in thin film solar cells. Nano Energy 2014, 6, 167–172. [Google Scholar] [CrossRef]
- Saravanan, M.; Dhivakar, S.; Jayanthi, S.S. An eco friendly and solvent free method for the synthesis of Zinc oxide nano particles using glycerol as organic dispersant. Mater. Lett. 2012, 67, 128–130. [Google Scholar] [CrossRef]
- Tuoc, V.N.; Huan, T.D.; Thao, N.T. Computational predictions of zinc oxide hollow structures. Phys. B Condens. Matter 2018, 532, 15–19. [Google Scholar] [CrossRef]
- Znajdek, K.; Sibiński, M.; Lisik, Z.; Apostoluk, A.; Zhu, Y.; Masenelli, B.; Sędzicki, P. Zinc oxide nanoparticles for improvement of thin film photovoltaic structures’ efficiency through down shifting conversion. Opto-Electron. Rev. 2017, 25, 99–102. [Google Scholar] [CrossRef]
- Bowland, C.C.; Malakooti, M.H.; Sodano, H.A. Barium titanate film interfaces for hybrid composite energy harvesters. ACS Appl. Mater. Interfaces 2017, 9, 4057–4065. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Sencadas, V.; Correia, V.; Rocha, J.G.; Lanceros-Mendez, S. Energy harvesting performance of piezoelectric electrospun polymer fibers and polymer/ceramic composites. Sens. Actuators A Phys 2013, 196, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-I.; Lee, G.-Y.; Yang, J.; Kim, C.-S.; Ahn, S.-H. Flexible ceramic-elastomer composite piezoelectric energy harvester fabricated by additive manufacturing. J. Compos. Mater. 2016, 50, 1573–1579. [Google Scholar] [CrossRef]
- Tuff, W.; Manghera, P.; Tilghman, J.; Fossen, E.; Chowdhury, S.; Ahmed, S.; Banerjee, S. BaTiO 3 –Epoxy–ZnO-Based Multifunctional Composites: Variation in Electron Transport Properties due to the Interaction of ZnO Nanoparticles with the Composite Microstructure. J. Electron. Mater. 2019, 48, 4987–4996. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, G. Electronic properties of a graphene/periodic porous graphene heterostructure. Carbon 2017, 122, 281–286. [Google Scholar] [CrossRef]
- Zaminpayma, E.; Razavi, M.E.; Nayebi, P. Electronic properties of graphene with single vacancy and Stone-Wales defects. Appl. Surf. Sci. 2017, 414, 101–106. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, S.; Kumar, R.; Shunmugam, R. Improved thermal and mechanical properties of polynorbornene upon covalent attachment with graphene sheets. Polymer 2017, 123, 321–333. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Ouadil, B.; Cherkaoui, O.; Safi, M.; Zahouily, M. Surface modification of knit polyester fabric for mechanical, electrical and UV protection properties by coating with graphene oxide, graphene and graphene/silver nanocomposites. Appl. Surf. Sci. 2017, 414, 292–302. [Google Scholar] [CrossRef]
- Anirudhan, T.; Deepa, J. Nano-zinc oxide incorporated graphene oxide/nanocellulose composite for the adsorption and photo catalytic degradation of ciprofloxacin hydrochloride from aqueous solutions. J. Colloid Interface Sci. 2017, 490, 343–356. [Google Scholar] [CrossRef]
- Banerjee, S.; Cook-Chennault, K.A. Influence of aluminium inclusions on dielectric properties of three-phase PZT–cement–aluminium composites. Adv. Cem. Res. 2014, 26, 63–76. [Google Scholar] [CrossRef]
- Guo, R.; Cross, L.; Park, S.; Noheda, B.; Cox, D.; Shirane, G. Origin of the high piezoelectric response in PbZr 1− x Ti x O 3. Phys. Rev. Lett. 2000, 84, 5423. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase. Compos. Part B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Marlinda, A.R.; Huang, N.M.; Muhamad, M.R.; An’Amt, M.N.; Chang, B.Y.S.; Yusoff, N.; Harrison, I.; Lim, H.N.; Chia, C.H.; Kumar, S.V. Highly efficient preparation of ZnO nanorods decorated reduced graphene oxide nanocomposites. Mater. Lett. 2012, 80, 9–12. [Google Scholar] [CrossRef]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Lombardi, M.; Landini, L.; Simi, S. Biological effects and safety in magnetic resonance imaging: A review. Int. J. Environ. Res. Public Health 2009, 6, 1778–1798. [Google Scholar] [CrossRef] [Green Version]
- Lieu, D. Ultrasound physics and instrumentation for pathologists. Arch. Pathol. Lab. Med. 2010, 134, 1541–1556. [Google Scholar]
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. MRI from Picture to Proton; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Szabo, T.L.; Lewin, P.A. Ultrasound transducer selection in clinical imaging practice. J. Ultrasound Med. 2013, 32, 573–582. [Google Scholar] [CrossRef] [PubMed]











| Material | Application | Property |
|---|---|---|
| Germanium/Silicon NW circuit [9,10] | Artificial skin | Low voltage operation flexible and small size |
| Silicon-based impedance sensor [11] | Detection of cancer cells | Large interaction sites Good electric signal transduction |
| SWCNTs on PDMS [9,12] | Human motion detection | High durability, fast response |
| Graphene aptameter [13] | Biosensor for thrombin | Low detection limit Fast charge transfer |
| Carbon Nanotube impedance biosensor [14] | Detecting DNA | Ultralow detection limit |
| Epoxy-Graphite impedimetric aptasensor [15] | Biosensor for cytochrome c | Low detecting limit High sensitivity |
| ZnO-glucose oxidase [16] | Detecting glucose | Good response time High sensitivity, low detection limit |
| CuO nanoleafs-ZnO NRs [17] | Glucose sensor | Good electrocatalytic property Low detection limit, low working potential |
| Pd/NF–ZnO-Graphene [18] | Glucose detection | High sensitivity, good catalytic activity, stability |
| ZnO-MnHCNF [19] | Riboflavin detection | Good electrocatalytic activity, sensitivity, stability |
| Ag-CuO nanocomposite [20] | Glucose detection | Good sensitivity & selectivity Fast electron transfer, fast response time |
| CuO-ZnO nanocomposite [21] | Glucose detection | Good electrocatalytic property Fast sensing towards glucose oxidation |
| VF of ZnO | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 |
|---|---|---|---|---|---|---|---|
| ZnO (g) | 2.523 | 5.0454 | 7.5681 | 10.090 | 12.614 | 15.136 | 17.659 |
| Resin (mL) | 3.574 | 3.177 | 2.779 | 2.382 | 1.985 | 1.588 | 1.191 |
| Hardener (mL) | 0.467 | 0.424 | 0.371 | 0.318 | 0.265 | 0.212 | 0.159 |
| Graphene (g) | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 |
| Ethanol (mL) | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Kumar, S.; Zoghi, S.; Cervantes, Y.; Sarkar, D.; Ahmed, S.; Chowdhury, S.; Banerjee, S. Fabrication and Characterization of Flexible Three-Phase ZnO-Graphene-Epoxy Electro-Active Thin-Film Nanocomposites: Towards Applications in Wearable Biomedical Devices. J. Compos. Sci. 2020, 4, 88. https://doi.org/10.3390/jcs4030088
Singh M, Kumar S, Zoghi S, Cervantes Y, Sarkar D, Ahmed S, Chowdhury S, Banerjee S. Fabrication and Characterization of Flexible Three-Phase ZnO-Graphene-Epoxy Electro-Active Thin-Film Nanocomposites: Towards Applications in Wearable Biomedical Devices. Journal of Composites Science. 2020; 4(3):88. https://doi.org/10.3390/jcs4030088
Chicago/Turabian StyleSingh, Mandeep, Sanjeev Kumar, Shervin Zoghi, Yerli Cervantes, Debaki Sarkar, Saquib Ahmed, Shaestagir Chowdhury, and Sankha Banerjee. 2020. "Fabrication and Characterization of Flexible Three-Phase ZnO-Graphene-Epoxy Electro-Active Thin-Film Nanocomposites: Towards Applications in Wearable Biomedical Devices" Journal of Composites Science 4, no. 3: 88. https://doi.org/10.3390/jcs4030088
APA StyleSingh, M., Kumar, S., Zoghi, S., Cervantes, Y., Sarkar, D., Ahmed, S., Chowdhury, S., & Banerjee, S. (2020). Fabrication and Characterization of Flexible Three-Phase ZnO-Graphene-Epoxy Electro-Active Thin-Film Nanocomposites: Towards Applications in Wearable Biomedical Devices. Journal of Composites Science, 4(3), 88. https://doi.org/10.3390/jcs4030088