Gap Junctions and Connexins in Physiology, Pharmacology and Disease
A topical collection in Biology (ISSN 2079-7737). This collection belongs to the section "Biochemistry and Molecular Biology".
Viewed by 48362Editors
Interests: intercellular communication; adipocyte, gap junction; energy metabolism; mitochondria; FGF21; hyaluronan; extracellular matrix; glycocalyx
Interests: electrophysiology; patch-clamp electrophysiology; synapses; neurophysiology; neurobiology
Interests: connexin 36; electrical synapses; tight junctions; gap junctions; connexins; PDZ domains; Rodentia; oligodendroglia
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
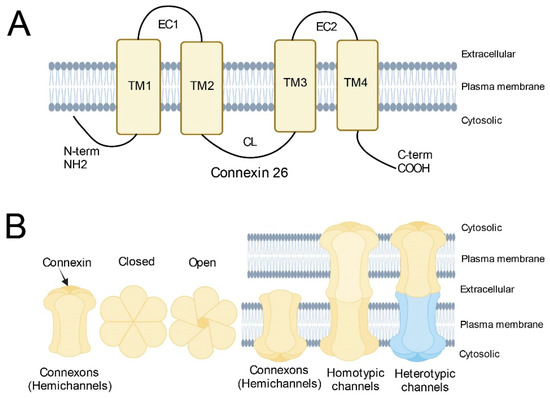
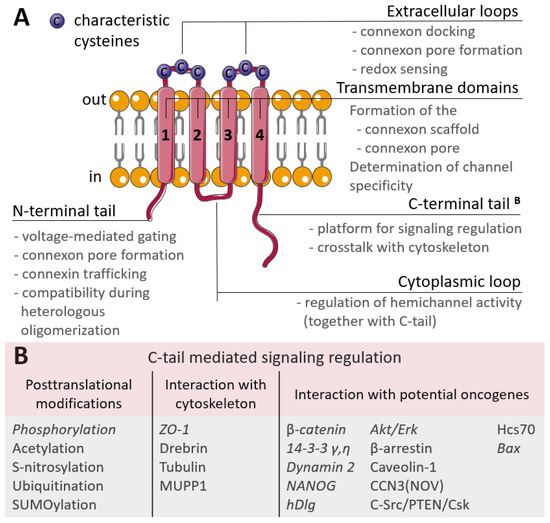
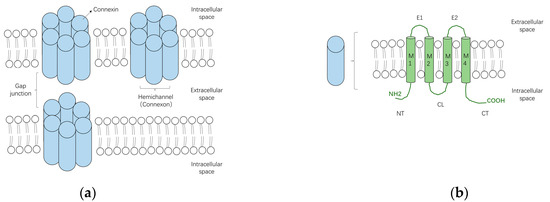
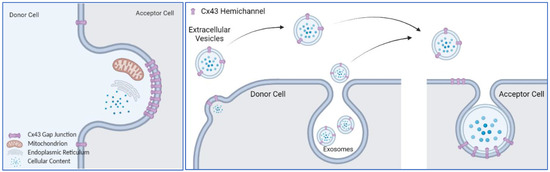
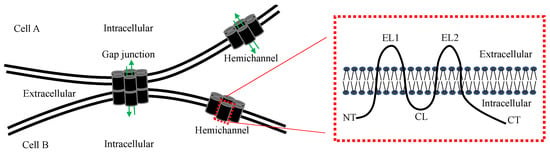
A characteristic feature of many cell types that make up the human body, is their extensive connection by gap junctions, which consist of a family of 21 highly heterogeneously expressed connexin (genes) proteins that form membrane channels allowing direct cell-to-cell exchange of water, amino acids, nucleotides, ATP, calcium, cAMP, IP3, and other small molecules with molecular weight less than 1,000 dalton. Gap junctions play pivotal roles for cell differentiation, proliferation, homeostasis, electrical synaptic transmission and whole organ function. Connexins can form gap junction channels as well as hemichannels, recently, emerging evidence also demonstrated that connexins can have gap junction channel-independent function.
Gap junction family, which includes connexins, pannexins and innexins in invertebrates. Due to the highly conserved sequence in mouse orthologs of their corresponding human connexins, gene knockout mice have been used to define the functions of connexin genes, and importantly, many connexins global knockout mice are showing a lethal phenotype, indicating the functional importance of gap junctions in maintaining the homeostasis of cells and tissues. Mutations in connexin genes have been associated with a variety of human disease conditions. For example, Cx26 gene mutations have been identified as the most prevalent cause of congenital hearing loss in children. Therefore, some clinical tests in connexin gene mutations have been widely used in medical diagnosis.
To date, the revolutionary Cryo-EM has become a powerful tool in studying membrane proteins, and the 3-D structures of Cx26, Cx46 and Cx50 proteins have been resolved using Cryo-EM. From a structural point of view, the reconstruction of all the connexin transmembrane proteins will provide new clue for elucidation of the functions or binding sites of specific gap junction channel as well as hemichannel blockers.
In gap junction field, by utilizing the advanced techniques of molecular biology and biochemistry, new tools, including, but not limited to CRISPR-Cas9 genome editing, base editing, prime editing, Cre/loxP conditional knockout mice, optogenetics, siRNA, shRNA, nanoparticle mediated gene delivery system, modified safe and high efficacy viral vectors for gene therapy, peptide blockage and Cryo-EM, may open up new avenues for the development of therapeutics based on blockage or activation of gap junctions. In this Topical Collection, the roles of gap junctions in normal physiology, pharmacology and in the pathogenesis of several diseases will be presented.
Dr. Yi Zhu
Dr. Sebastian Curti
Dr. Xinbo Li
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biology is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- gap junctions
- connexins
- pannexins
- innexins
- cell communication
- cell–cell coupling
- mutation
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.
Title: Post-translational Modifications and Multi-site Control of Connexin43
Authors: Corbin Glufka; Jeffery Li; Vincent Chen
Affiliation: Department of Chemistry, Brandon University, Room 4-11, John R. Brodie Science Centre, 270 18th Street, Brandon, Manitoba, Canada
Abstract: Post-translational modifications (PTMs) are dynamic chemical events that control and fine tune the function of a cell. Occurring at any point within the life cycle of a protein, the presence or absence of a PTMs can profoundly influence gene function and thus regulatory events underlying health and disease. The identification binary and combinatorial PTMs such as phosphorylation are of wide interest to the gap junction field. In this review we will discuss the current state of knowledge of multi-site PTMs and how these modifications maybe accessed to help determine their effects on connexin structure, function, and gap junctional intercellular communication.
Title: Connexin36 interacting proteins
Authors: Xinbo Li1; Rongqiang Li2
Affiliation: 1. Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, USA
2. Department of Urology, Weihaiwei hospital, Weihai, Shandong, China
Abstract: It is well established that gap junction protein connexin36 (Cx36) is one of the major components of the morphological structure of electrical synapses in the central nervous system. Recent emerging evidence indicating that Cx36 contains a highly conserved C-terminus SAYV PDZ domain binding motif, and the SAYV PDZ domain binding motif is required for the interaction of Cx36 with a variety of PDZ domain containing proteins. In this review, we will summarize the current progress in studying Cx36 associated proteins, including our recently discovered the Rho-guanine nucleotide exchange factor PDZ-RhoGEF, and their functional and regulatory roles in the formation of electrical synapses comprised of Cx36.