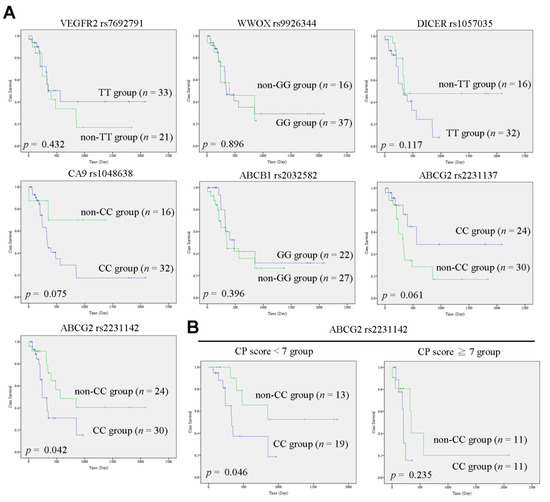
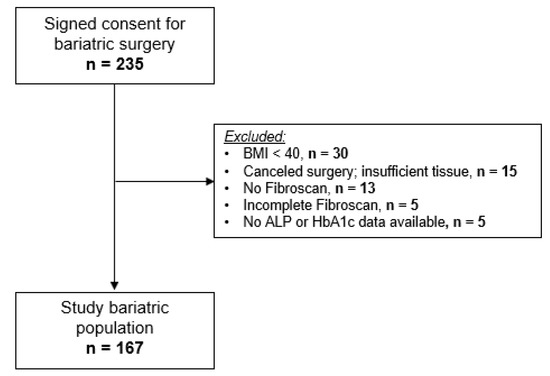
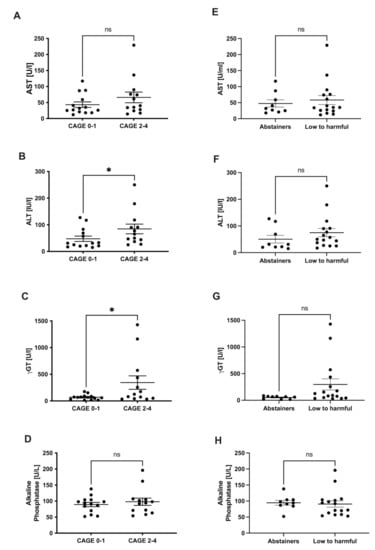
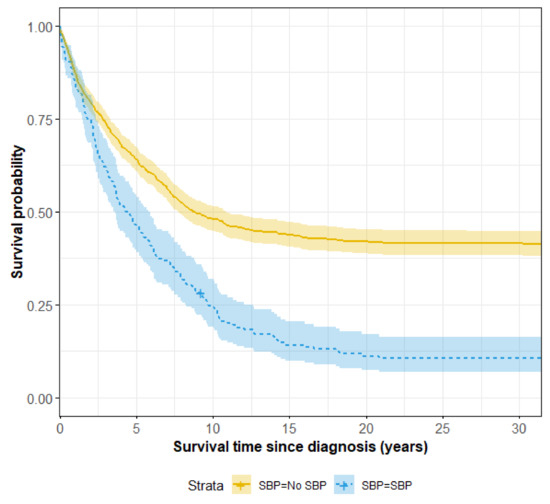
Clinical Research in Hepatology
A topical collection in Journal of Clinical Medicine (ISSN 2077-0383). This collection belongs to the section "Gastroenterology & Hepatopancreatobiliary Medicine".
Viewed by 63916Editor
2. Department of Internal Medicine III, University Hospital RWTH Aachen, Aachen, Germany
Interests: metabolic and alcoholic associated fatty liver diseases; liver fibrosis; HCC; DILI
Topical Collection Information
Dear Colleagues,
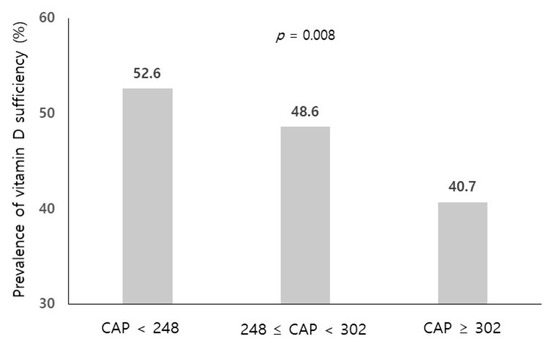
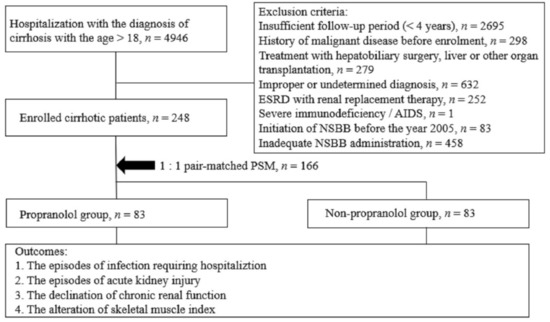
The spectrum of etiologies for liver diseases (LD) is broad and includes toxins, alcohol abuse, infection, autoimmune diseases, and genetic and metabolic disorders. In general, LD accounts annually for approximately 2 million deaths worldwide, due to complications of cirrhosis, liver cancer, or deterioration of liver functions. Besides that, the economic impact is high and the quality of life is low in patients with LD. Though this is sobering, it also highlights an important opportunity to improve public health, given that most causes of liver diseases are preventable. Indeed, over the past 30 years, remarkable progress has been made in basic/clinical research, vaccination programs, drug discovery, and development in the field of hepatology.
In this Topical Collection of JCM, we invite you to contribute original and cutting-edge research articles, reviews, letters, or case studies related to the theme of “Clinical research in hepatology”. We encourage submissions from international contributors and researchers of all specialties that relate to clinical hepatology and liver diseases. Relevant topics include but are not limited to: NASH, NAFLD, ALD, hepatitis, DILI, hepatic fibrosis, steatosis, HCC, cholangiocarcinoma, rare liver diseases, inherited hepatic disorders, treatment and management of LD complications, therapeutic perspectives, and translational aspects in hepatology.
Prof. Dr. Yulia A. Nevzorova
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Clinical Medicine is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- NAFLD
- ALD
- HCC
- CCC
- Hepatitis Cirrhosis
- DILI
- Liver transplantation
- Clinical hepatology