Function, Regulation, and Dysfunction of Intrinsically Disordered Proteins (Closed)
A topical collection in Life (ISSN 2075-1729). This collection belongs to the section "Proteins and Proteomics".
Viewed by 66867Editors
Interests: biochemistry; structural biology; functional intrinsically disordered proteins; parkinson; alzheimer
Special Issues, Collections and Topics in MDPI journals
Interests: protein folding; protein dynamics and disorder; protein-protein recognition
Special Issues, Collections and Topics in MDPI journals
Interests: intrinsically disordered proteins; folding copuled to binding; protein-protein interactions; structural transitions; paramyxoviruses
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
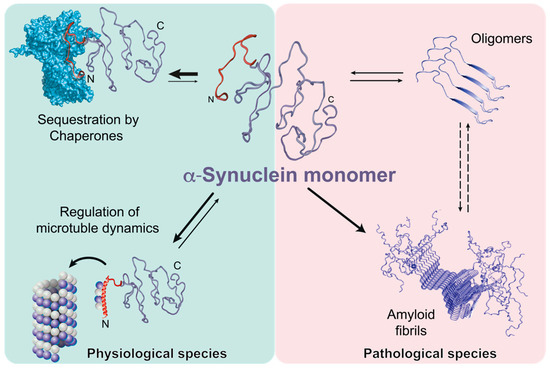
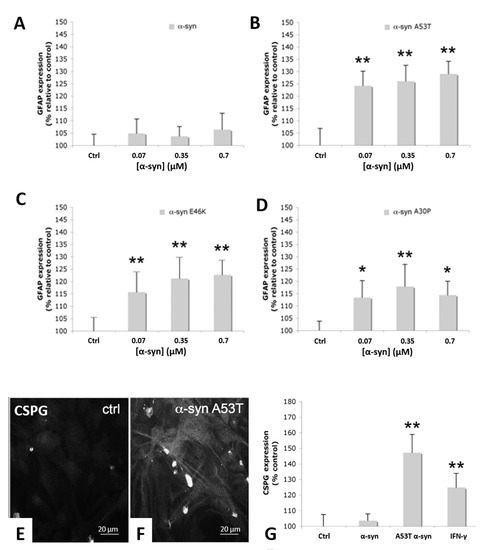
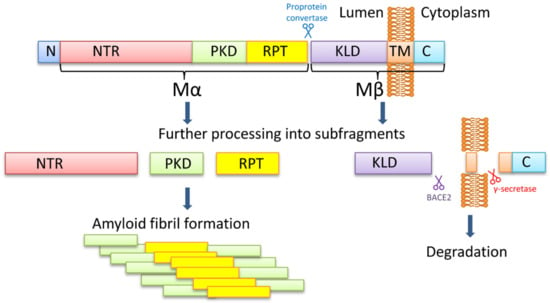
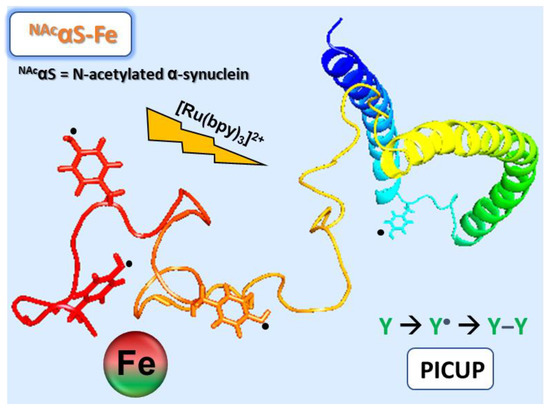
It has become acknowledged that a significant portion of proteins encoded in eukaryotic and prokaryotic genomes feature a partial or total degree of structural disorder. Disordered regions can regulate the biological activity of protein molecules, such as the propensity to generate molecular complexity, including the regulation of phase separations. The involvement of intrinsically disordered proteins in fundamental biological processes, including cellular signaling, protein translation, and transcriptional regulation, is increasingly reported to be crucial in functional and pathological mechanisms. The remarkable ability of these molecules to establish macromolecular interactions with multiple biomolecular partners is likely promoted by their inherent flexibility and ability to adapt their shape. While the functional role of IDPs is attracting an increasing research focus, understanding the underlying structural and mechanistic principles of their biological activity remains a crucial research challenge. The role of kinetic versus thermodynamic control is the key to understand the way by which these elusive systems are regulated to solve their intriguing biological functions.
Dr. Giuliana Fusco
Prof. Dr. Stefano Gianni
Dr. Sonia Longhi
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Life is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- intrinsically disordered proteins
- post-translational modifications
- phase separation
- structural and functional dynamics
- binding promiscuity and fuzzy complexes
- molten globular states
- kinetic vs thermodynamic control
- conformational selection vs induced fit
Related Special Issue
2021
Jump to: 2020
2020
Jump to: 2021
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.