Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterisation
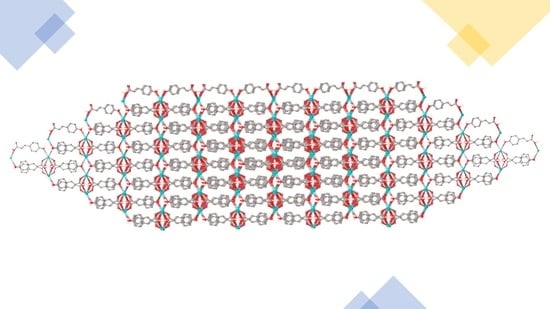
2.2. X-ray Crystal Structures
2.2.1. M(3tpc) Complexes (1M = 1Li, 1K, 1Rb, 1Cs)
2.2.2. M(2m3fur) Complexes (2M = 2Li, 2K)
2.2.3. 2D Polymeric Chain of the [Li(3fur)]n (3Li) Complex
2.2.4. M(4hocin) Complexes (4M = 4K, 4Rb, 4Cs)
2.2.5. M(4hob) Complexes (5M = 5Li, 5K, 5Rb, 5Cs)
2.3. Corrosion Inhibition
Immersion Tests
3. Materials and Methods
3.1. General Consideration
3.2. X-ray Crystallography
3.3. Synthesis of Alkali-Metal Carboxylate Complexes
3.4. Corrosion Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineeering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Markley, T.; Blin, F.; Forsyth, M.; Hinton, B. Multifunctional Rare Earth Organic Corrosion Inhibitors. In Rare Earth-Based Corrosion Inhibitors; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 117–142. ISBN 9780857093585. [Google Scholar]
- Somers, A.E.; Hinton, B.R.W.; de Bruin-Dickason, C.; Deacon, G.B.; Junk, P.C.; Forsyth, M. New, Environmentally Friendly, Rare Earth Carboxylate Corrosion Inhibitors for Mild Steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Forsyth, M.; Seter, M.; Hinton, B.; Deacon, G.; Junk, P. New “Green” Corrosion Inhibitors Based on Rare Earth Compounds. Aust. J. Chem. 2011, 64, 812–819. [Google Scholar] [CrossRef]
- Seter, M.; Girard, G.M.A.; Lee, W.W.; Deacon, G.; Junk, P.; Hinton, B.; Forsyth, M. The Influence of Organic Structure and Rare Earth Metal Cation on the Corrosion Efficiency Observed on AS1020 Steel Compared with La(4OHCin)3. AIMS Mater. Sci. 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Somers, A.E.; Peng, Y.; Chong, A.L.; Forsyth, M.; MacFarlane, D.R.; Deacon, G.B.; Hughes, A.E.; Hinton, B.R.W.; Mardel, J.I.; Junk, P.C. Advances in the Development of Rare Earth Metal and Carboxylate Compounds as Corrosion Inhibitors for Steel. Corros. Eng. Sci. Technol. 2020, 55, 311–321. [Google Scholar] [CrossRef]
- Somers, A.E.; Deacon, G.B.; Hinton, B.R.W.; Macfarlane, D.R.; Junk, P.C.; Tan, M.Y.J.; Forsyth, M. Recent Developments in Environment-Friendly Corrosion Inhibitors for Mild Steel. J. Indian Inst. Sci. 2016, 96, 285–292. [Google Scholar]
- Behrsing, T.; Deacon, G.B.; Junk, P.C. The Chemistry of Rare Earth Metals, Compounds, and Corrosion Inhibitors. In Rare Earth-Based Corrosion Inhibitors; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 1–37. [Google Scholar]
- De Damborenea, J.; Conde, A.; Arenas, M.A. Corrosion Inhibition with Rare Earth Metal Compounds in Aqueous Solutions. In Rare Earth-Based Corrosion Inhibitors; Woodhead Publishing: Cambridge, UK, 2014; pp. 84–116. [Google Scholar] [CrossRef]
- Forsyth, M.; Wilson, K.; Behrsing, T.; Forsyth, C.; Deacon, G.B.; Phanasgoankar, A. Effectiveness of Rare-Earth Metal Compounds as Corrosion Inhibitors for Steel. Corrosion 2002, 58, 953–960. [Google Scholar] [CrossRef]
- Blin, F.; Leary, S.G.; Deacon, G.B.; Junk, P.C.; Forsyth, M. The Nature of the Surface Film on Steel Treated with Cerium and Lanthanum Cinnamate Based Corrosion Inhibitors. Corros. Sci. 2006, 48, 404–419. [Google Scholar] [CrossRef]
- Peng, Y.; Hughes, A.E.; Deacon, G.B.; Junk, P.C.; Hinton, B.R.W.; Forsyth, M.; Mardel, J.I.; Somers, A.E. A Study of Rare-Earth 3-(4-Methylbenzoyl)-Propanoate Compounds as Corrosion Inhibitors for AS1020 Mild Steel in NaCl Solutions. Corros. Sci. 2018, 145, 199–211. [Google Scholar] [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Somers, A.E.; Junk, P.C. RE(III) 3-Furoate Complexes: Synthesis, Structure, and Corrosion Inhibiting Properties. Molecules 2022, 27, 8836. [Google Scholar] [CrossRef]
- Vithana, V.P.; Guo, Z.; Deacon, G.B.; Somers, A.E.; Junk, P.C. Synthesis, Structure, and Corrosion Inhibiting Properties of REIII 3-Thiophenecarboxylate Complexes. New J. Chem. 2022, 46, 19104–19111. [Google Scholar] [CrossRef]
- Moon, J.S.; Guo, Z.; Beaumont, O.A.; Hamilton, S.; Bousrez, G.; Mottram, E.; Wang, J.; Somers, A.E.; Deacon, G.B.; Junk, P.C. 4-Hydroxybenzoato-Rare Earth(III) Complexes—Syntheses, Structures, Coordination Diversity and Corrosion Inhibition Behaviour. New J. Chem. 2023, submitted.
- Ouchi, A.; Suzuki, Y.; Ohki, Y.; Koizumi, Y. Structure of Rare Earth Carboxylates in Dimeric and Polymeric Forms. Coord. Chem. Rev. 1988, 92, 29–43. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of Rare Earth Elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Blin, F.; Leary, S.G.; Wilson, K.; Deacon, G.B.; Junk, P.C.; Forsyth, M. Corrosion Mitigation of Mild Steel by New Rare Earth Cinnamate Compounds. J. Appl. Electrochem. 2004, 34, 591–599. [Google Scholar] [CrossRef]
- Wormwell, F.; Mercer, A.D. Sodium Benzoate and Other Metal Benzoates as Corrosion-Inhibitors in Water and in Aqueous Solutions. J. Appl. Chem. 1952, 2, 150–160. [Google Scholar] [CrossRef]
- Forsyth, M.; Forsyth, C.M.; Wilson, K.; Behrsing, T.; Deacon, G.B. ATR Characterisation of Synergistic Corrosion Inhibition of Mild Steel Surfaces by Cerium Salicylate. Corros. Sci. 2002, 44, 2651–2656. [Google Scholar] [CrossRef]
- Mercer, A.D. The Properties of Carboxylates as Corrosion Inhibitors for Steel and Other Metals in Neutral Aqueous Solutions. In Proceedings of the 5th European Symposium on Corrosion Inhibitors; University of Ferrara: Ferrara, Italy, 1980; pp. 563–581. [Google Scholar]
- ConQuest 2.0.4. Version 5.41; ConQuest: Cambridge, UK, 2023.
- Dinnebier, R.E.; Dreele, R.; Stephens, P.W.; Jelonek, S.; Sieler, J. Structure of Sodium Para-Hydroxybenzoate, NaO 2 C±C 6 H 4 OH by Powder Diffraction: Application of a Phenomenological Model of Anisotropic Peak Width. J. Appl. Cryst. 1999, 32, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Skinner, J.M.; Speakman, J.C. The Crystal Structures of the Acid Salts of Some Monobasic Acids. Part II. Potassium Hydrogen Di-p-Hydroxybenzoate Hydrate. J. Chem. Soc. 1951, 185–191. [Google Scholar] [CrossRef]
- Manojlovi, L. A Reinvestigation of the Crystal Structure of Potassium Hydrogen Di-p-Hydroxybenzoate Hydrate. Acta Cryst. 1968, 24, 326. [Google Scholar] [CrossRef]
- Hanusa, T.P. Group 1s and 2s Metals. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.B., Eds.; Elsevier: Oxford, UK, 2004; Volume 3, pp. 1–92. [Google Scholar]
- Koby, R.F.; Hanusa, T.P. Lithium, Sodium, Potassium, Rubidium, and Cesium. Compr. Coord. Chem. III 2021, 1–9, 2–48. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy; Thompson Learning, Inc.: Novato, CA, USA, 2001. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Dai, F.R.; Qiao, Y.; Wang, Z. Designing Structurally Tunable and Functionally Versatile Synthetic Supercontainers. Inorg. Chem. Front. 2016, 3, 243–249. [Google Scholar] [CrossRef]
- Mustapha, A.; Reglinski, J.; Kennedy, A.R. Metal Complexes as Potential Ligands: The Deprotonation of Aminephenolate Metal Complexes. Inorg. Chem. Commun. 2010, 13, 464–467. [Google Scholar] [CrossRef] [Green Version]
- Deacon, G.B.; Forsyth, C.M.; Harika, E.; Junk, P.C.; Ziller, J.W.; Evans, W.J. Hydrocarbon-Soluble, Polymetallic, Lanthanoid Aryloxides Constructed Utilising Ligands with Distal But Groups. J. Mater. Chem. 2004, 14, 3144–3149. [Google Scholar] [CrossRef]
- Dai, F.R.; Wang, Z. Modular Assembly of Metal-Organic Supercontainers Incorporating Sulfonylcalixarenes. J. Am. Chem. Soc. 2012, 134, 8002–8005. [Google Scholar] [CrossRef]
- Demaison, J.; Herman, M.; Lievin, J. The Equilibrium OH Bond Length. Int. Rev. Phys. Chem. 2007, 26, 391–420. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.Q. Assembly of 3D Metal-Organic Framework Based on Heterobimetallic Cu-K Unit and Oxalate Linkage. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2010, 40, 237–240. [Google Scholar] [CrossRef]
- Yuan, F.; Zhou, Y.; Li, L.; Qian, H. Synthesis and Crystal Structure of a Trinuclear Aminobis(Phenolate) Ytterbium Hydroxide Complex. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 46, 913–916. [Google Scholar] [CrossRef]
- Mehring, M.; Nolde, C.; Schürmann, M. Octameric Sodium Trimethylsilanolate Hemihydrate Hemi-THF Solvate, [NaOSiMe3·1/2H2O·1/2THF]8. Appl. Organomet. Chem. 2004, 18, 487–488. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Agilent Technologies. CrysAlisPRO v.39; Agilent Technologies Ltd.: Yarnton, UK, 2013. [Google Scholar]
- McPhillips, T.M.; McPhillips, S.E.; Chiu, H.J.; Cohen, A.E.; Deacon, A.M.; Ellis, P.J.; Garman, E.; Gonzalez, A.; Sauter, N.K.; Phizackerley, R.P.; et al. Blu-Ice and the Distributed Control System: Software for Data Acquisition and Instrument Control at Macromolecular Crystallography Beamlines. J. Synchrotron Radiat. 2002, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. Automatic Processing of Rotation Diffraction Data from Crystals of Initially Unknown Symmetry and Cell Constants. J. Appl. Crystallogr. 1993, 26, 795–800. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM: West Conshohocken, PA, USA, 2004.
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A.; et al. MX1: A Bending-Magnet Crystallography Beamline Serving Both Chemical and Macromolecular Crystallography Communities at the Australian Synchrotron. J. Synchrotron Radiat. 2015, 22, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]






























| Compounds | Significant Bands (cm−1) | ||||
|---|---|---|---|---|---|
| ν(OH) | n(C=C) Propenyl | νas(CO2−) | νs(CO2−) | Δν a | |
| [Li2(3tpc)2]n 1Li | - | - | 1566,1514 | 1414,1399 | 133.5 |
| [K2(3tpc)2]n 1K | - | - | 1558,1516 | 1412,1401 | 130.5 |
| [Rb(3tpc)(H2O)]n 1Rb | 3348 | - | 1563,1512 | 1405 | 132.5 |
| [Cs{H(3tpc)2}]n 1Cs | - | - | 1560,1510 | 1398,1384 | 144 |
| [Li2(2m3fur)2(H2O)3] 2Li | 3301,3373 | - | 1544,1521 | 1428 | 104.5 |
| [K2(2m3fur)2(H2O)]n 2K | 3354 | - | 1532,1517 | 1418 | 106.5 |
| [Li(3fur)]n 3Li | - | - | 1552 | 1428 | 124 |
| [K(4hocin)(H2O)3]n 4K | 3114,3444 | 1636 | 1531,1511 | 1371 | 150 |
| [Rb{H(4hocin)2}]n·nH2O 4Rb | 3241 | 1634 | 1537,1514 | 1381 | 144.5 |
| [Cs(4hocin)(H2O)]n 4Cs | 3200 | 1633 | 1537,1514 | 1378 | 147.5 |
| [Li(4hob)]n 5Li | 3351 | - | 1556 | 1399 | 157 |
| [K(4hob)(H2O)3]n 5K | 3437 | - | 1533 | 1361 | 172 |
| [Rb(4hob)(H2O)]n 5Rb | 3317 | - | 1529,1509 | 1381 | 138 |
| [Cs(4hob)(H2O)]n 5Cs | 3202 | - | 1529,1509 | 1376 | 143 |
| Compound | M Atom | Geometry | Coordination Number | Ligation | Avg M-O (Å) |
|---|---|---|---|---|---|
| 1Li | Li1 Li2 | Tetrahedral Tetrahedral | 4 4 | 4 × (i) 4 × (i) | 1.963 1.963 |
| 1K | K1 K2 | Irregular polyhedron Irregular polyhedron | 5 5 | 1 × (ii) a; 3 × (ii) b 1 × (ii) a; 3 × (ii) b | 2.791 2.767 |
| 1Rb | Rb1 | Distorted capped trigonal prismatic | 7 | 3 × (iii); 4 × µ4-H2O | 2.995 |
| 1Cs | Cs1 | Cubic | 8 | 8 × (i) | 3.152 |
| 2Li | Li1 | Tetrahedral | 4 | 2 × (iv); 1 × H2O; 2 × µ2-H2O | 1.957 |
| 2K | K1 | Distorted pseudo-pentagonal bipyramidal | 8 | 1 × (ii) a; 3 × (v) b; 1 × (ii) b; 2 × µ3-H2O | 2.841 |
| K2 | Distorted pseudo-square pyramidal | 6 | 1 × (v) a; 2 × (ii) b; 1 × (v) b; 1 × µ3-H2O | 2.750 | |
| 3Li | Li1 | Tetrahedral | 4 | 4 × (i) | 1.978 |
| 4K | K1 | Distorted tricapped trigonal prismatic | 9 | 2 × (vi); 3 × µ3-H2O; 2 × µ2-H2O; 2 × µ2-H2O; | 2.977 |
| 4Rb | Rb1 | Irregular polyhedron | 6 | 4 × (vii); 2 × phenolic O | 2.932 |
| 4Cs | Cs1 | Irregular polyhedron | 8 | 1 × (viii) a; 2 × (viii) b; 2 × µ2-H2O; 2 × phenolic O | 3.349 |
| 5Li | Li1 | Tetrahedral | 4 | 4 × (i) | 1.953 |
| 5K | K1 | Square antiprismatic | 8 | 2 × (vi); 1 × H2O; 3 × µ3-H2O; 2 × µ2-H2O | 2.912 |
| 5Rb | Rb1 | Square antiprismatic | 8 | 4 × (i); 2 × µ2-H2O; 2 × phenolic O | 3.049 |
| 5Cs | Cs1 | Square antiprismatic | 8 | 4 × (i); 2 × µ2-H2O; 2 × phenolic O | 3.217 |
| Solution | Concentration | Corrosion Rate (μgm−2s−1) | % Inhibition (η) | |
|---|---|---|---|---|
| ppm | mM | |||
| Control—NaCl | 580 | 10 | 39.1 | - |
| [Li2(3tpc)2]n (1Li) | 800 | 2.98 | 27.1 | 31 |
| [K2(3tpc)2]n (1K) | 800 | 2.41 | 28.8 | 26 |
| [Rb(3tpc)(H2O)]n (1Rb) | 800 | 3.47 | 27.4 | 30 |
| [K2(2m3fur)2(H2O)]n (2K) | 800 | 2.31 | 35.7 | 9 |
| [Li(3fur)]n (3Li) | 800 | 6.78 | 29.2 | 25 |
| [K(4hocin)(H2O)3]n (4K) | 800 | 3.12 | 7.5 | 80 |
| [K(4hob)(H2O)3]n (5K) | 800 | 3.47 | 29.7 | 24 |
| Ligand | Solutions | Concentration | Corrosion Rate (μgm−2s−1) | % Inhibition (η) | Ref | |
|---|---|---|---|---|---|---|
| ppm | mM | |||||
| 1-3tpc | [La(3tpc)3(H2O)3]n | 500 | 0.871 | 19.4 | 36 | [14] |
| 1-3tpc | [Ce(3tpc)3(H2O)3]n | 500 | 0.869 | 18.8 | 38 | [14] |
| 1-3tpc | [Y2(3tpc)6(H2O)4]. H2O | 500 | 0.485 | 9.65 | 68 | [14] |
| 3-3fur | [La(3fur)3(H2O)2]n | 800 | 1.57 | 5.14 | 87 | [13] |
| 3-3fur | [Ce(3fur)3(H2O)2]n | 800 | 1.57 | 8.3 | 79 | [13] |
| 3-3fur | [Y(3fur)3(H2O)2]n | 800 | 1.75 | 3.9 | 90 | [13] |
| 4-4ohcin | La(4hocin)3 | 500 | 0.7 | 3.2 | 91 | [18] |
| 4-4ohcin | Ce(4hocin)3 | 200 | 0.28 | 5.7 | 84 | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vithana, V.P.; Guo, Z.; Deacon, G.B.; Junk, P.C. Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes. Molecules 2023, 28, 5515. https://doi.org/10.3390/molecules28145515
Vithana VP, Guo Z, Deacon GB, Junk PC. Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes. Molecules. 2023; 28(14):5515. https://doi.org/10.3390/molecules28145515
Chicago/Turabian StyleVithana, Vidushi P., Zhifang Guo, Glen B. Deacon, and Peter C. Junk. 2023. "Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes" Molecules 28, no. 14: 5515. https://doi.org/10.3390/molecules28145515
APA StyleVithana, V. P., Guo, Z., Deacon, G. B., & Junk, P. C. (2023). Syntheses, Structures, and Corrosion Inhibition of Various Alkali Metal Carboxylate Complexes. Molecules, 28(14), 5515. https://doi.org/10.3390/molecules28145515