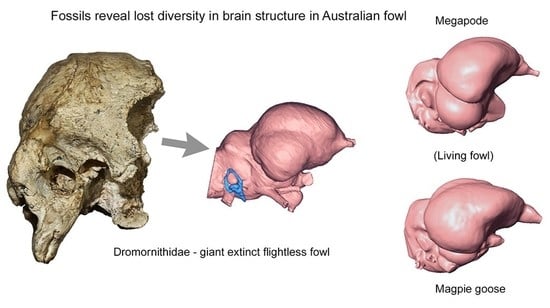
Endocranial Anatomy of the Giant Extinct Australian Mihirung Birds (Aves, Dromornithidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abbreviations
Institutions
2.2. Geological and Temporal Data for Fossil Specimens
2.3. Nomenclature
2.4. Modelling
2.4.1. Three-Dimensional (3D) Surface Model Construction
2.4.2. Model Reconstructions
2.4.3. Remeshing
2.5. Landmarking
2.6. Data
2.6.1. Modular Lm Data
2.6.2. Measurement Data
2.6.3. Surface Area Data
2.7. Analyses
2.7.1. Generalized Procrustes Analysis (GPA)
2.7.2. Three-Dimensional Modular Shape Variation Plots
3. Results
3.1. Dromornithid Innervation That Differs from the Extant Galloanseres (see Figure 3 and Figure 4; SI Figures S4 and S5)
3.1.1. Nervus Olfactorius
3.1.2. Nervus Opticus
3.1.3. Nervus Trigeminus
3.1.4. Nervus Abducens
3.1.5. Nervus Glossopharyngeus
3.1.6. Nervus Hypoglossus
3.2. Characteristics of Dromornithid Endocast Morphology
3.2.1. Rostral Telencephalon
3.2.2. Wulst
3.2.3. Caudal Telencephalon
3.2.4. Optic Lobe
3.2.5. Trigeminal Ganglia
3.2.6. Cerebellum
3.2.7. Rhombencephalon
3.3. Key Morphological Differences between Species of Dromornis and Ilbandornis
3.3.1. Wulst Modules
3.3.2. Caudal Telencephalon Modules
3.3.3. Cerebellum Module
3.3.4. Rhombencephalon Module
3.4. Key Morphological Differences between Dromornithids and the Extant Galloanseres
3.4.1. Innervation
3.4.2. Wulst Modules
3.4.3. Rostral Telencephalon Modules
3.4.4. Caudal Telencephalon Modules
3.4.5. Optic Lobe Modules
3.4.6. Cerebellum Module
3.4.7. Rhombencephalon Module
4. Discussion
4.1. Comparisons of Endocranial Characteristics of Dromornithids and Extant Galloanseres
4.1.1. Olfactory Bulb
4.1.2. Trigeminal Ganglia
4.1.3. Wulst
4.1.4. Rostral Telencephalon
4.1.5. Caudal Telencephalon
4.1.6. Optic Lobe
4.1.7. Cerebellum
4.1.8. Rhombencephalon
4.2. Endocranial Morphology Distinguishing Lineages within Dromornithids
4.3. Temporal Changes in the Endocranial Morphology of the Dromornis Lineage
4.4. Functional Implications of Dromornithid Endocranial Morphology
4.4.1. Innervation
4.4.2. Visual Pathways
4.4.2.1. Wulst
4.4.2.2. Cerebrum
4.4.2.3. Optic Lobe
4.4.2.4. Hindbrain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vickers-Rich, P. The Mesozoic and Tertiary History of Birds on the Australian Plate. In Vertebrate Palaeontology of Australasia; Vickers-Rich, P., Monaghan, J.M., Baird, R.F., Rich, T.H., Eds.; Pioneer Design Studios and Monash University Publications Committee: Melbourne, Australia, 1991; pp. 721–808. [Google Scholar]
- Murray, P.F.; Vickers-Rich, P. Magnificent Mihirungs: The Colossal Flightless Birds of the Australian Dreamtime; Indiana University Press: Bloomington, IN, USA, 2004; p. 410. [Google Scholar]
- Murray, P.F.; Megirian, D. The skull of dromornithid birds: Anatomical evidence for their relationship to Anseriformes. Rec. South Aust. Mus. 1998, 31, 51–97. [Google Scholar]
- Murray, P.F.; Megirian, D. The Pwerte Marnte Marnte Local Fauna: A new vertebrate assemblage of presumed Oligocene age from the Northern Territory of Australia. Alcheringa 2006, 30, 211–228. [Google Scholar] [CrossRef]
- Boles, W.E. The Avian Fossil Record of Australia: An Overview. In Evolution and Biogeography of Australasian Vertebrates; Merrick, J.R., Archer, M., Hickey, G.M., Lee, M.S.Y., Eds.; Auscipub: Sydney, Australia, 2006; pp. 387–411. [Google Scholar]
- Worthy, T.H.; Handley, W.D.; Archer, M.; Hand, S.J. The extinct flightless mihirungs (Aves, Dromornithidae): Cranial anatomy, a new species, and assessment of Oligo-Miocene lineage diversity. J. Vertebr. Paleontol. 2016, 36, e1031345. [Google Scholar] [CrossRef]
- Vickers-Rich, P.; Molnar, R.E. The foot of a bird from the Eocene Redbank Plains Formation of Queensland, Australia. Alcheringa 1996, 20, 21–29. [Google Scholar] [CrossRef]
- Mayr, G. Paleogene Fossil Birds; Springer: Berlin, Germany, 2009; p. 262. [Google Scholar]
- Saltré, F.; Rodríguez-Rey, M.; Brook, B.W.; Johnson, C.N.; Turney, C.S.; Alroy, J.; Cooper, A.; Beeton, N.; Bird, M.I.; Fordham, D.A. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 2016, 7, 10511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, R. [Part 19 of Owen’s memoir on Dinornis, read June 4, 1872]. P. Zool. Soc. Lond. 1872, 1872, 682–683. [Google Scholar]
- Rich, P.V. The Dromornithidae, an extinct family of large ground birds endemic to Australia. Bur. Nat. Resour. Geol. Geophys. Bull. 1979, 184, 1–194. [Google Scholar]
- Nguyen, J.M.T.; Boles, W.E.; Hand, S.J. New material of Barawertornis tedfordi, a dromornithid bird from the Oligo-Miocene of Australia, and its phylogenetic implications. Rec. Aust. Mus. 2010, 62, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Worthy, T.H.; Yates, A. Connecting the thigh and foot: Resolving the association of post-cranial elements in the species of Ilbandornis (Aves: Dromornithidae). Alcheringa 2015, 39, 1–21. [Google Scholar] [CrossRef]
- Stirling, E.C.; Zietz, A.H.C. Preliminary notes on Genyornis newtoni: A new genus and species of fossil struthious bird found at Lake Callabonna, South Australia. T. Roy. Soc. South Aust. 1896, 20, 171–211. [Google Scholar]
- Wells, R.T.; Tedford, R.H. Sthenurus (Macropodidae: Marsupialia) from the Pleistocene of Lake Callabonna, South Australia. B. Am. Mus. Nat. Hist. 1995, 225, 3–111. [Google Scholar]
- Stirling, E.C.; Zietz, A.H.C. Genyornis newtoni—A fossil struthious bird from Lake Callabonna, South Australia: Description of the bones of the leg and foot. T. Roy. Soc. South Aust. 1896, 20, 191–211. [Google Scholar]
- Wetmore, A. A classification for the birds of the world. Smithson. Misc. Collect. 1960, 139, 1–37. [Google Scholar]
- Rich, P.V. Changing continental arrangements and the origin of Australia’s non-passeriform continental avifauna. Emu Austral Ornithol. 1975, 75, 97–112. [Google Scholar] [CrossRef]
- Worthy, T.H.; Holdaway, R.N. The Lost World of the Moa: Prehistoric Life of New Zealand; Indiana University Press: Bloomington, IA, USA, 2002; p. 760. [Google Scholar]
- Phillips, M.J.; Gibb, G.C.; Crimp, E.A.; Penny, D. Tinamous and moa flock together: Mitochondrial genome sequence analysis reveals independent losses of flight among ratites. Syst. Biol. 2010, 59, 90–107. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.L. The Fossil Record of Birds. In Avian Biology; Farner, D.S., King, J.R., Parkes, K.C., Eds.; Academic Press: New York, NY, USA, 1985; Volume 8, pp. 79–238. [Google Scholar]
- Mayr, G. Cenozoic mystery birds–on the phylogenetic affinities of bony-toothed birds (Pelagornithidae). Zool. Scr. 2011, 40, 448–467. [Google Scholar] [CrossRef]
- Worthy, T.H.; Mitri, M.; Handley, W.D.; Lee, M.S.Y.; Anderson, A.; Sand, C. Osteology supports a stem-Galliform affinity for the giant extinct flightless bird Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PLoS ONE 2016, 11, e0150871. [Google Scholar] [CrossRef] [Green Version]
- Worthy, T.H.; Degrange, F.J.; Handley, W.D.; Lee, M.S.Y. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). Roy. Soc. Open Sci. 2017, 4, 170975. [Google Scholar] [CrossRef] [Green Version]
- Worthy, T.H.; Degrange, F.J.; Handley, W.D.; Lee, M.S.Y. Correction to ‘The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres)’. Roy. Soc. Open Sci. 2017, 4, 171621. [Google Scholar] [CrossRef] [PubMed]
- Andors, A.V. Reappraisal of the Eocene groundbird Diatryma (Aves: Anserimorphae). Nat. Hist. Mus. Los Angeles County Sci. Ser. 1992, 36, 109–125. [Google Scholar]
- Angst, D.; Lécuyer, C.; Amiot, R.; Buffetaut, E.; Fourel, F.; Martineau, F.; Legendre, S.; Abourachid, A.; Herrel, A. Isotopic and anatomical evidence of an herbivorous diet in the Early Tertiary giant bird Gastornis. Implications for the structure of Paleocene terrestrial ecosystems. Naturwissenschaften 2014, 101, 313–322. [Google Scholar] [CrossRef]
- Archer, M.; Godthelp, H.; Hand, S.J.; Attenborough, D. Australia’s Lost World: Riversleigh, World Heritage Site; New Holland Publishers: Sydney, Austrilia, 1991; p. 264. [Google Scholar]
- Woodburne, M.O. The Alcoota Fauna, central Australia: An integrated palaeontological and geological study. B. Bur. Min. Res. Geol. Geophy. 1967, 87, 1–187. [Google Scholar]
- Murray, P.F.; Megirian, D. Continuity and contrast in middle and late Miocene vertebrate communities from the Northern Territory. Rec. Mus. Art Gall. Northern Terr. 1992, 9, 195–218. [Google Scholar]
- Handley, W.D.; Chinsamy, A.; Yates, A.M.; Worthy, T.H. Sexual dimorphism in the late Miocene mihirung Dromornis stirtoni (Aves: Dromornithidae) from the Alcoota Local Fauna of central Australia. J. Vertebr. Paleontol. 2016, 36, e1180298. [Google Scholar] [CrossRef]
- Grellet-Tinner, G.; Spooner, N.A.; Handley, W.D.; Worthy, T.H. The Genyornis egg: Response to Miller et al.’s commentary on Grellet-Tinner et al., 2016. Quaternary Sci. Rev. 2017, 161, 128–133. [Google Scholar] [CrossRef]
- Hansford, J.P.; Turvey, S.T. Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world’s largest bird. Roy. Soc. Open Sci. 2018, 5, 181295. [Google Scholar] [CrossRef] [Green Version]
- Archer, M.; Godthelp, H.; Hand, S.J.; Megirian, D. Fossil mammals of Riversleigh, northwestern Queensland: Preliminary overview of biostratigraphy, correlation and environmental change. Austr. Zool. 1989, 25, 29–65. [Google Scholar] [CrossRef] [Green Version]
- Archer, M.; Hand, S.J.; Godthelp, H.; Creaser, P. Correlation of the Cainozoic sediments of the Riversleigh World Heritage fossil property, Queensland, Australia. In Actes du Congrès BiochroM ‘97. Mémoires Travaux Inst. Montp. 1997, 21, 131–152. [Google Scholar]
- Travouillon, K.J.; Archer, M.; Hand, S.J.; Godthelp, H. Multivariate analyses of Cenozoic mammalian faunas from Riversleigh, northwestern Queensland. Alcheringa Spec. Iss. 2006, 1, 323–349. [Google Scholar] [CrossRef]
- Travouillon, K.J.; Escarguel, G.; Legendre, S.; Archer, M.; Hand, S.J. The use of MSR (Minimum Sample Richness) for sample assemblage comparisons. Paleobiology 2011, 37, 696–709. [Google Scholar] [CrossRef]
- Woodhead, J.; Hand, S.J.; Archer, M.; Graham, I.; Sniderman, K.; Arena, D.A.; Black, K.H.; Godthelp, H.; Creaser, P.; Price, E. Developing a radiometrically-dated chronologic sequence for Neogene biotic change in Australia, from the Riversleigh World Heritage Area of Queensland. Gondwana Res. 2016, 29, 153–167. [Google Scholar] [CrossRef]
- Arena, D.A.; Travouillon, K.J.; Beck, R.M.D.; Black, K.H.; Gillespie, A.K.; Myers, T.J.; Archer, M.; Hand, S.J. Mammalian lineages and the biostratigraphy and biochronology of Cenozoic faunas from the Riversleigh World Heritage Area, Australia. Lethaia 2016, 49, 43–60. [Google Scholar] [CrossRef]
- Megirian, D.; Prideaux, G.J.; Murray, P.F.; Smit, N. An Australian land mammal age biochronological scheme. Paleobiology 2010, 36, 658–671. [Google Scholar] [CrossRef]
- Murray, P.; Megirian, D.; Rich, T.; Plane, M.; Black, K.; Archer, M.; Hand, S.J.; Vickers-Rich, P. Morphology, systematics, and evolution of the marsupial genus Neohelos Stirton (Diprotodontidae, Zygomaturinae). Mus. Gall. Northern Terr. Res. Rep. 2000, 6, 1–141. [Google Scholar]
- Yates, A.M. New craniodental remains of Wakaleo alcootaensis (Diprotodontia: Thylacoleonidae) a carnivorous marsupial from the late Miocene Alcoota Local Fauna of the Northern Territory, Australia. PeerJ 2015, 3, e1408. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.M. A new species of long-necked turtle (Pleurodira: Chelidae: Chelodina) from the late Miocene Alcoota Local Fauna, Northern Territory, Australia. PeerJ 2013, 1, e170. [Google Scholar] [CrossRef]
- Worthy, T.H.; Yates, A. A review of the smaller birds from the late Miocene Alcoota Local Faunas of Australia with a description of a new species. In Proceedings of the 9th International Meeting of the Society of Avian Paleontology and Evolution, Diamante, Argentina, 1–6 August 2016. Contr. Mus. Arg. Cien. Nat. 2017, 7, 221–252. [Google Scholar]
- Yates, A.M.; Worthy, T.H. A diminutive species of emu (Casuariidae: Dromaiinae) from the late Miocene of the Northern Territory, Australia. J. Vertebr. Paleontol. 2019, 39, e1665057. [Google Scholar] [CrossRef]
- Stirton, R.A.; Woodburne, M.O.; Plane, M.D. A phylogeny of the Tertiary Diprotodontidae and its significance in correlation. In Tertiary Diprotodontidae from Australia and New Guinea. B. Bur. Min. Res. Geol. Geophy. 1967, 85, 149–160. [Google Scholar]
- Baumel, J.J.; King, A.S.; Breazile, J.E.; Evans, H.E.; Vanden Berge, J.C. Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed.; Nuttall Ornithological Club: Cambridge, MA, USA, 1993; Volume 23, p. 779. [Google Scholar]
- Jarvis, E.D.; Güntürkün, O.; Bruce, L.; Csillag, A.; Karten, H.; Kuenzel, W.; Medina, L.; Paxinos, G.; Perkel, D.J.; Shimizu, T. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005, 6, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corfield, J.R.; Wild, J.M.; Parsons, S.; Kubke, M.F. Morphometric analysis of telencephalic structure in a variety of neognath and paleognath bird species reveals regional differences associated with specific behavioral traits. Brain Behav. Evolut. 2012, 80, 181–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early, C.M.; Iwaniuk, A.N.; Ridgely, R.C.; Witmer, L.M. Endocast structures are reliable proxies for the sizes of corresponding regions of the brain in extant birds. J. Anat. 2020, 237, 1162–1176. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U.S. National Institute of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 28 January 2021).
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. Year Meshlab: An open-source mesh processing tool. In Eurographics Italian Chapter Conference; Scarano, V., De Chiara, R., Erra, U., Eds.; The Eurographics Association: Geneve, Switzerland, 2008; pp. 129–136. [Google Scholar] [CrossRef]
- Wiley, D.F. Landmark Editor 3.0; Institute for Data Analysis and Visualization, University of California: Davis, CA, USA, 2006. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991; p. 435. [Google Scholar]
- Adams, D.C.; Collyer, M.L.; Kaliontzopoulou, A. Geomorph: Software for Geometric Morphometric Analyses. R Package Version 3.1.0. 2019. Available online: https://cran.r-project.org/src/contrib/Archive/geomorph/ (accessed on 25 March 2019).
- Mosimann, J.E. Size allometry: Size and shape variables with characterizations of the lognormal and generalized gamma distributions. J. Am. Stat. Assoc. 1970, 65, 930–945. [Google Scholar] [CrossRef]
- Claude, J. Morphometrics with R; Springer: New York, NY, USA, 2008; p. 316. [Google Scholar]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, E.; Vidal-García, M.; Anstis, M.; Keogh, J.S. Adult frogs and tadpoles have different macroevolutionary patterns across the Australian continent. Nat. Ecol. Evol. 2017, 1, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 28 January 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 28 January 2021).
- Gower, J.C. Generalized Procrustes analysis. Psychometrika 1975, 40, 33–51. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Bookstein, F.L. Size and shape spaces for landmark data in two dimensions. Stat. Sci. 1986, 1, 181–242. [Google Scholar] [CrossRef]
- Adams, D.C.; Otárola-Castillo, E. geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Bookstein, F.L. Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar] [CrossRef]
- Bookstein, F.L. Shape and the information in medical images: A decade of the morphometric synthesis. Comput. Vis. Image Und. 1997, 2, 97–118. [Google Scholar] [CrossRef] [Green Version]
- Iwaniuk, A.N.; Nelson, J.E. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 2002, 80, 16–23. [Google Scholar] [CrossRef]
- Striedter, G.F. Principles of Brain Evolution; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Striedter, G.F. Précis of principles of brain evolution. Behav. Brain Sci. 2006, 29, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Witmer, L.M.; Ridgely, R.C.; Dufeau, D.L.; Semones, M.C. Using CT to Peer into the Past: 3D Visualization of the Brain and Ear Regions of Birds, Crocodiles, and Nonavian Dinosaurs. In Anatomical Imaging, Towards a New Morphology; Endo, H., Frey, R., Eds.; Springer: Tokyo, Japan, 2008; pp. 67–87. [Google Scholar]
- Picasso, M.B.J.; Tambussi, C.; Dozo, M.T. Neurocranial and brain anatomy of a Late Miocene eagle (Aves, Accipitridae) from Patagonia. J. Vertebr. Paleontol. 2009, 29, 831–836. [Google Scholar] [CrossRef]
- Walsh, S.A.; Iwaniuk, A.N.; Knoll, M.A.; Bourdon, E.; Barrett, P.M.; Milner, A.C.; Nudds, R.L.; Abel, R.L.; Sterpaio, P.D. Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS ONE 2013, 8, e67176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.A.; Knoll, F. The Evolution of Avian Intelligence and Sensory Capabilities: The Fossil Evidence. In Digital Endocasts: From Skulls to Brains; Bruner, E., Ogihara, N., Tanabe, H.C., Eds.; Springer: Tokyo, Japan, 2018; pp. 59–69. [Google Scholar]
- Scofield, R.P.; Ashwell, K.W.S. Rapid somatic expansion causes the brain to lag behind: The case of the brain and behavior of New Zealand’s Haast’s Eagle (Harpagornis moorei). J. Vertebr. Paleontol. 2009, 29, 637–649. [Google Scholar] [CrossRef]
- Lawal, R.A.; Al-Atiyat, R.M.; Aljumaah, R.S.; Silva, P.; Mwacharo, J.M.; Hanotte, O. Whole-genome resequencing of red junglefowl and indigenous village chicken reveal new insights on the genome dynamics of the species. Front. Genet. 2018, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwell, K.W.S.; Scofield, R.P. Big birds and their brains: Paleoneurology of the New Zealand moa. Brain Behav. Evolut. 2008, 71, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Craigie, E.H. The cerebral cortex of Rhea americana. J. Comp. Neurol. 1939, 70, 331–353. [Google Scholar] [CrossRef]
- Martin, G.R.; Wilson, K.-J.; Wild, J.M.; Parsons, S.; Kubke, M.F.; Corfield, J. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2007, 2, e198. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Feng, Y.; Zhang, G.; Liu, H.; Song, H. Anatomical study of the brain of the African ostrich. Turk. J. Vet. Anim. Sci. 2010, 34, 235–241. [Google Scholar] [CrossRef]
- Picasso, M.B.J.; Tambussi, C.P.; Degrange, F.J. Virtual reconstructions of the endocranial cavity of Rhea americana (Aves, Palaeognathae): Postnatal anatomical changes. Brain Behav. Evolut. 2011, 76, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Witmer, L.M.; Chatterjee, S.; Franzosa, J.; Rowe, T. Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature 2003, 425, 950–953. [Google Scholar] [CrossRef]
- Milner, A.C.; Walsh, S.A. Avian brain evolution: New data from Palaeogene birds (Lower Eocene) from England. Zool. J. Linn. Soc. Lond. 2009, 155, 198–219. [Google Scholar] [CrossRef] [Green Version]
- Witmer, L.M.; Ridgely, R.C. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat. Rec. 2009, 292, 1266–1296. [Google Scholar] [CrossRef]
- Walsh, S.A.; Luo, Z.-X.; Barrett, P.M. Modern Imaging Techniques as a Window to Prehistoric Auditory Worlds. In Insights from Comparative Hearing Research; Koppl, C., Manley, G.A., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2014; pp. 227–261. [Google Scholar]
- Hall, M.I.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C. Optic foramen morphology and activity pattern in birds. Anat. Rec. 2009, 292, 1827–1845. [Google Scholar] [CrossRef]
- Jerison, H.J. Evolution of the Brain and Intelligence; Academic Press: New York, NY, USA, 1973; p. 482. [Google Scholar]
- Barton, R.A.; Purvis, A.; Harvey, P.H. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philos. T. Roy. Soc. B. 1995, 348, 381–392. [Google Scholar]
- Barton, R.A.; Aggleton, J.P.; Grenyer, R. Evolutionary coherence of the mammalian amygdala. P. Roy. Soc. B. Biol. Sci. 2003, 270, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Barton, R.A.; Harvey, P.H. Mosaic evolution of brain structure in mammals. Nature 2000, 405, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Whiting, B.A.; Barton, R.A. The evolution of the cortico-cerebellar complex in primates: Anatomical connections predict patterns of correlated evolution. J. Hum. Evolut. 2003, 44, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Iwaniuk, A.N.; Dean, K.M.; Nelson, J.E. A mosaic pattern characterizes the evolution of the avian brain. P. Roy. Soc. B. Biol. Sci. 2004, 271, S148–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubbeldam, J.L. Birds. In The Central Nervous System of Vertebrates; Nieuwenhuys, R., ten Donkelaar, H.J., Nicholson, C., Eds.; Springer: Berlin, Germany, 1998; pp. 1525–1636. [Google Scholar]
- Iwaniuk, A.N.; Heesy, C.P.; Hall, M.I.; Wylie, D.R. Relative wulst volume is correlated with orbit orientation and binocular visual field in birds. J. Comp. Physiol. A. 2008, 194, 267–282. [Google Scholar] [CrossRef]
- Corfield, J.R.; Price, K.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Birkhead, T.; Wylie, D.R. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Front. Neuroanat. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ibáñez, C.; Iwaniuk, A.N.; Wylie, D.R. The independent evolution of the enlargement of the principal sensory nucleus of the trigeminal nerve in three different groups of birds. Brain Behav. Evolut. 2009, 74, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Dubbeldam, J.L. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. II. Morphology of the principal sensory nucleus. J. Comp. Neurol. 1980, 191, 557–571. [Google Scholar] [CrossRef]
- Bubień-Waluszewska, A. The Cranial Nerves. In Form and Function in Birds; King, A.S., McLelland, J., Eds.; Academic Press: London, UK, 1981; Volume 2, pp. 385–438. [Google Scholar]
- Dubbeldam, J.L.; Brauch, C.S.M.; Don, A. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. III. Afferents and organization of the nucleus basalis. J. Comp. Neurol. 1981, 196, 391–405. [Google Scholar] [CrossRef]
- Wild, J.M.; Zeigler, H.P. Central projections and somatotopic organisation of trigeminal primary afferents in pigeon (Columba livia). J. Comp. Neurol. 1996, 368, 136–152. [Google Scholar] [CrossRef]
- Dubbeldam, J.L.; Brus, E.R.; Menken, S.B.J.; Zeilstra, S. The central projections of the glossopharyngeal and vagus ganglia in the mallard, Anas platyrhynchos L. J. Comp. Neurol. 1979, 183, 149–168. [Google Scholar] [CrossRef]
- Wild, J.M.; Zeigler, H.P. Central representation and somatotopic organization of the jaw muscles within the facial and trigeminal nuclei of the pigeon (Columba livia). J. Comp. Neurol. 1980, 192, 175–201. [Google Scholar] [CrossRef]
- Wild, J.M. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus, and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. J. Comp. Neurol. 1981, 203, 351–377. [Google Scholar] [CrossRef]
- Wild, J.M. Peripheral and central terminations of hypoglossal afferents innervating lingual tactile mechanoreceptor complexes in Fringillidae. J. Comp. Neurol. 1990, 298, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Dubbeldam, J.L. The sensory trigeminal system in birds: Input, organization and effects of peripheral damage. Arch. Physiol. Biochem. 1998, 106, 338–345. [Google Scholar] [CrossRef]
- Wild, J.M. The avian somatosensory system: Connections of regions of body representation in the forebrain of the pigeon. Brain Res. 1987, 412, 205–223. [Google Scholar] [CrossRef]
- Dubbeldam, J.L. Cranial nerves and sensory centres―A matter of definition? Hypoglossal and other afferents of the avian sensory trigeminal system. Zool. Jahrb. 1992, 122, 179–186. [Google Scholar]
- Dubbeldam, J.L. Afferent connections of nervus facialis and nervus glossopharyngeus in the pigeon (Columba livia) and their role in feeding behavior. Brain Behav. Evolut. 1984, 24, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.J.A.; Dubbeldam, J.L. The subnuclei and primary afferents of the descending trigeminal system in the mallard (Anas platyrhynchos L.). Neuroscience 1984, 13, 781–795. [Google Scholar] [CrossRef]
- Davies, S.J.J.F. The food of emus. Austral Ecol. 1978, 3, 411–422. [Google Scholar] [CrossRef]
- Davies, S.J.J.F. Bird Families of the World: Ratites and Tinamous: Tinamidae, Rheidae, Dromaiidae, Casuariidae, Apterygidae, Struthionidae; Oxford University Press: Oxford, UK, 2002; p. 310. [Google Scholar]
- Wings, O. A review of gastrolith function with implications for fossil vertebrates and a revised classification. Acta Palaeontol. Pol. 2007, 52, 1–16. [Google Scholar]
- Fritz, J.; Hummel, J.; Kienzle, E.; Wings, O.; Streich, W.J.; Clauss, M. Gizzard vs. teeth, it’s a tie: Food-processing efficiency in herbivorous birds and mammals and implications for dinosaur feeding strategies. Paleobiology 2011, 37, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Worthy, T.H. Aspects of the biology of two moa species (Aves: Dinornithiformes). New Zeal. J. Arch. 1989, 11, 77–86. [Google Scholar]
- Wood, J.R.; Rawlence, N.J.; Rogers, G.M.; Austin, J.J.; Worthy, T.H.; Cooper, A. Coprolite deposits reveal the diet and ecology of the extinct New Zealand megaherbivore moa (Aves, Dinornithiformes). Quat. Sci. Rev. 2008, 27, 2593–2602. [Google Scholar] [CrossRef]
- Wood, J.R.; Wilmshurst, J.M.; Richardson, S.J.; Rawlence, N.J.; Wagstaff, S.J.; Worthy, T.H.; Cooper, A. Resolving lost herbivore community structure using coprolites of four sympatric moa species (Aves: Dinornithiformes). Pro. Natl. Acad. Sci. USA 2013, 110, 16910–16915. [Google Scholar] [CrossRef] [Green Version]
- Wylie, D.R.; Gutiérrez-Ibáñez, C.; Pakan, J.M.P.; Iwaniuk, A.N. The optic tectum of birds: Mapping our way to understanding visual processing. Can. J. Exp. Psychol. 2009, 63, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Pakan, J.M.P.; Wylie, D.R. Allometric scaling of the tectofugal pathway in birds. Brain Behav. Evolut. 2010, 75, 122–137. [Google Scholar] [CrossRef]
- Wylie, D.R.; Iwaniuk, A.N. Neural Mechanisms Underlying Visual Motion Detection in Birds. In How Animals See the World: Comparative Behavior, Biology, and Evolution of Vision; Lazareva, O.F., Shimizu, T., Wasserman, E.A., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 289–318. [Google Scholar]
- Iwaniuk, A.N.; Wylie, D.R. The evolution of stereopsis and the wulst in caprimulgiform birds: A comparative analysis. J. Comp. Physiol. A 2006, 192, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Williams, M.N. Rostral wulst in passerine birds. I. Origin, course, and terminations of an avian pyramidal tract. J. Comp. Neurol. 2000, 416, 429–450. [Google Scholar] [CrossRef]
- Miceli, D.; Marchand, L.; Repérant, J.; Rio, J.-P. Projections of the dorsolateral anterior complex and adjacent thalamic nuclei upon the visual wulst in the pigeon. Brain Res. 1990, 518, 317–323. [Google Scholar] [CrossRef]
- Deng, C.; Wang, B. Overlap of somatic and visual response areas in the wulst of pigeon. Brain Res. 1992, 582, 320–322. [Google Scholar]
- Stingelin, W. Vergleichende Morphologische Untersuchungen am Vorderhirn der Vögel auf Cytologischer und Cytoarchitektonischer Grundlage; Verlag Helbing & Lichtenhahn: Basel, Switzerland, 1957; p. 123. [Google Scholar]
- Pettigrew, J.D. Evolution of Binocular Vision. In Visual Neuroscience; Pettigrew, J.D., Sanderson, K.J., Levick, W.R., Eds.; Springer: New York, NY, USA, 1986; pp. 208–222. [Google Scholar]
- Rogers, L. Behavioral, structural and neurochemical asymmetries in the avian brain: A model system for studying visual development and processing. Neurosci. Biobehav. Rev. 1996, 20, 487–503. [Google Scholar] [CrossRef]
- Wild, J.M.; Kubke, M.F.; Peña, J.L. A pathway for predation in the brain of the barn owl (Tyto alba): Projections of the gracile nucleus to the “claw area” of the rostral wulst via the dorsal thalamus. J. Comp. Neurol. 2008, 509, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Wild, J.M. The avian somatosensory system: The pathway from wing to wulst in a passerine (Chloris chloris). Brain Res. 1997, 759, 122–134. [Google Scholar] [CrossRef]
- Manger, P.R.; Elston, G.N.; Pettigrew, J.D. Multiple maps and activity-dependent representational plasticity in the anterior wulst of the adult barn owl (Tyto alba). Eur. J. Neurosci. 2002, 16, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettigrew, J.D.; Frost, B.J. A tactile fovea in the Scolopacidae? Brain Behav. Evolut. 1985, 26, 185–195. [Google Scholar] [CrossRef]
- Martin, G.R. What is binocular vision for? A birds’ eye view. J. Vision. 2009, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Wylie, D.R.; Gutiérrez-Ibáñez, C.; Iwaniuk, A.N. Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front. Neurosci. Switz. 2015, 9, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettigrew, J.D.; Konishi, M. Neurons selective for orientation and binocular disparity in the visual wulst of the barn owl (Tyto alba). Science 1976, 193, 675–678. [Google Scholar] [CrossRef]
- Pettigrew, J.D. Binocular visual processing in the owl’s telencephalon. Proc. Roy. Soc. B Biol. Sci. 1979, 204, 435–454. [Google Scholar] [CrossRef]
- Van der Willigen, R.F.; Frost, B.J.; Wagner, H. Stereoscopic depth perception in the owl. NeuroReport 1998, 9, 1233–1237. [Google Scholar] [CrossRef]
- Orlowski, J.; Harmening, W.; Wagner, H. Night vision in barn owls: Visual acuity and contrast sensitivity under dark adaptation. J. Vision. 2012, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Ibáñez, C.; Iwaniuk, A.N.; Lisney, T.J.; Wylie, D.R. Comparative study of visual pathways in owls (Aves: Strigiformes). Brain Behav. Evolut. 2013, 81, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kulemeyer, C.; Asbahr, K.; Gunz, P.; Frahnert, S.; Bairlein, F. Functional morphology and integration of corvid skulls–a 3D geometric morphometric approach. Front. Zool. 2009, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northcutt, R.G. Evolution of the telencephalon in nonmammals. Annu. Rev. Neurosci. 1981, 4, 301–350. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Arends, J.J.A.; Zeigler, H.P. Telencephalic connections of the trigeminal system in the pigeon (Columba livia): A trigeminal sensorimotor circuit. J. Comp. Neurol. 1985, 234, 441–464. [Google Scholar] [CrossRef]
- Hall, M.I.; Ross, C.F. Eye shape and activity pattern in birds. J. Zool. 2007, 271, 437–444. [Google Scholar] [CrossRef]
- Hall, M.I. The anatomical relationships between the avian eye, orbit and sclerotic ring: Implications for inferring activity patterns in extinct birds. J. Anat. 2008, 212, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, A.N.; Heesy, C.P.; Hall, M.I. Morphometrics of the eyes and orbits of the nocturnal swallow-tailed gull (Creagrus furcatus). Can. J. Zool. 2010, 88, 855–865. [Google Scholar] [CrossRef]
- Corfield, J.R.; Gsell, A.C.; Brunton, D.; Heesy, C.P.; Hall, M.I.; Acosta, M.L.; Iwaniuk, A.N. Anatomical specializations for nocturnality in a critically endangered parrot, the Kakapo (Strigops habroptilus). PLoS ONE 2011, 6, e22945. [Google Scholar] [CrossRef] [Green Version]
- Corfield, J.R.; Wild, J.M.; Hauber, M.E.; Parsons, S.; Kubke, M.F. Evolution of brain size in the palaeognath lineage, with an emphasis on New Zealand ratites. Brain Behav. Evolut. 2008, 71, 87–99. [Google Scholar] [CrossRef]
- Garamszegi, L.Z.; Møller, A.P.; Erritzøe, J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. Roy. Soc. B Biol. Sci. 2002, 269, 961–967. [Google Scholar] [CrossRef]
- Dubbeldam, J.L.; Visser, A.M. The organization of the nucleus basalis—neostriatum complex of the mallard (Anas platyrhynchos L.) and its connections with the archistriatum and the paleostriatum complex. Neuroscience 1987, 21, 487–517. [Google Scholar] [CrossRef]
- Reiner, A.; Davis, B.M.; Brecha, N.C.; Karten, H.J. The distribution of enkephalinlike immunoreactivity in the telencephalon of the adult and developing domestic chicken. J. Comp. Neurol. 1984, 228, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Salzen, E.A.; Parker, D.M.; Williamson, A.J. A forebrain lesion preventing imprinting in domestic chicks. Exp. Brain Res. 1975, 24, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, A.N.; Wylie, D.R. Sensory systems in birds: What we have learned from studying sensory specialists. J. Comp. Neurol. 2020, 528, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Berkhoudt, H.; Dubbeldam, J.L.; Zeilstra, S. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. IV. Tactile representation in the nucleus basalis. J. Comp. Neurol. 1981, 196, 407–420. [Google Scholar] [CrossRef]
- Schneider, E.R.; Mastrotto, M.; Laursen, W.J.; Schulz, V.P.; Goodman, J.B.; Funk, O.H.; Gallagher, P.G.; Gracheva, E.O.; Bagriantsev, S.N. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Pro. Natl. Acad. Sci. USA 2014, 111, 14941–14946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.R.; Anderson, E.O.; Mastrotto, M.; Matson, J.D.; Schulz, V.P.; Gallagher, P.G.; LaMotte, R.H.; Gracheva, E.O.; Bagriantsev, S.N. Molecular basis of tactile specialization in the duck bill. Pro. Natl. Acad. Sci. USA 2017, 114, 13036–13041. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.R.; Anderson, E.O.; Feketa, V.V.; Mastrotto, M.; Nikolaev, Y.A.; Gracheva, E.O.; Bagriantsev, S.N. A cross-species analysis reveals a general role for Piezo2 in mechanosensory specialization of trigeminal ganglia from tactile specialist birds. Cell Rep. 2019, 26, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Wild, J.M.; Arends, J.J.A.; Zeigler, H.P. A trigeminal sensorimotor circuit for pecking, grasping and feeding in the pigeon (Columba livia). Brain Res. 1984, 300, 146–151. [Google Scholar] [CrossRef]
- Wild, J.M.; Farabaugh, S.M. Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. J. Comp. Neurol. 1996, 365, 306–328. [Google Scholar] [CrossRef]
- Von Eugen, K.; Tabrik, S.; Güntürkün, O.; Ströckens, F. A comparative analysis of the dopaminergic innervation of the executive caudal nidopallium in pigeon, chicken, zebra finch, and carrion crow. J. Comp. Neurol. 2020, 528, 2929–2955. [Google Scholar] [CrossRef]
- Hellmann, B.; Güntürkün, O.; Manns, M. Tectal mosaic: Organization of the descending tectal projections in comparison to the ascending tectofugal pathway in the pigeon. J. Comp. Neurol. 2004, 472, 395–410. [Google Scholar] [CrossRef]
- Gibson, J.J. The visual perception of objective motion and subjective movement. Psychol. Rev. 1954, 61, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Wylie, D.R.; Gutiérrez-Ibáñez, C.; Gaede, A.H.; Altshuler, D.L.; Iwaniuk, A.N. Visual-cerebellar pathways and their roles in the control of avian flight. Front. Neurosci. Switz. 2018, 12, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, J.I. The accessory optic system. Annu. Rev. Neurosci. 1984, 7, 13–41. [Google Scholar] [CrossRef]
- Simpson, J.I.; Leonard, C.S.; Soodak, R.E. The accessory optic system. Analyzer of self-motion. Ann. N. Y. Acad. Sci. 1988, 545, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Giolli, R.A.; Blanks, R.H.; Lui, F. The accessory optic system: Basic organization with an update on connectivity, neurochemistry, and function. Prog. Brain Res. 2006, 151, 407–440. [Google Scholar]
- Gaede, A.H.; Gutiérrez-Ibáñez, C.; Armstrong, M.S.; Altshuler, D.L.; Wylie, D.R. Pretectal projections to the oculomotor cerebellum in hummingbirds (Calypte anna), zebra finches (Taeniopygia guttata), and pigeons (Columba livia). J. Comp. Neurol. 2019, 527, 2644–2658. [Google Scholar] [CrossRef]
- Pakan, J.M.P.; Wylie, D.R. Two optic flow pathways from the pretectal nucleus lentiformis mesencephali to the cerebellum in pigeons (Columba livia). J. Comp. Neurol. 2006, 499, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, A.N.; Keirnan, A.R.; Janetzki, H.; Mardon, K.; Murphy, S.; Leseberg, N.P.; Weisbecker, V. The endocast of the Night Parrot (Pezoporus occidentalis) reveals insights into its sensory ecology and the evolution of nocturnality in birds. Sci. Rep. 2020, 10, 9258. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.F.; Kirk, E.C. Osteological evidence for the evolution of activity pattern and visual acuity in primates. Am. J. Phys. Anthropol. 2000, 113, 235–262. [Google Scholar] [CrossRef]
- Bennett, P.M.; Harvey, P.H. Relative brain size and ecology in birds. J. Zool. 1985, 207, 151–169. [Google Scholar] [CrossRef]
- Lau, K.L.; Glover, R.G.; Linkenhoker, B.; Wylie, D.R. Topographical organization of inferior olive cells projecting to translation and rotation zones in the vestibulocerebellum of pigeons. Neuroscience 1998, 85, 605–614. [Google Scholar] [CrossRef]
- Wylie, D.R. Projections from the nucleus of the basal optic root and nucleus lentiformis mesencephali to the inferior olive in pigeons (Columba livia). J. Comp. Neurol. 2001, 429, 502–513. [Google Scholar] [CrossRef]
- Wylie, D.R. Processing of visual signals related to self-motion in the cerebellum of pigeons. Front. Behav. Neurosci. 2013, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaniuk, A.N.; Hurd, P.L.; Wylie, D.R. Comparative morphology of the avian cerebellum: II. Size of folia. Brain Behav. Evolut. 2007, 69, 196–219. [Google Scholar] [CrossRef]
- Walsh, S.A.; Knoll, M.A. Directions in palaeoneurology. Spec. Pap. Palaeontol. 2011, 86, 263–279. [Google Scholar] [CrossRef]
- Martin, H.A. Cenozoic climatic change and the development of the arid vegetation in Australia. J. Arid Environ. 2006, 66, 533–563. [Google Scholar] [CrossRef]
- Macphail, M.K. Late Neogene climates in Australia: Fossil pollen-and spore-based estimates in retrospect and prospect. Aust. J. Bot. 1997, 45, 425–464. [Google Scholar] [CrossRef]
- Stirling, E.C. The recent discovery of fossil remains at Lake Callabonna, South Australia II. Nature 1894, 50, 206–211. [Google Scholar]
- Hamm, G.; Mitchell, P.; Arnold, L.J.; Prideaux, G.J.; Questiaux, D.; Spooner, N.A.; Levchenko, V.A.; Foley, E.C.; Worthy, T.H.; Stephenson, B. Cultural innovation and megafauna interaction in the early settlement of arid Australia. Nature 2016, 539, 280–283. [Google Scholar] [CrossRef]
- Szabo, M.J. Stout-legged moa. New Zeal. Birds Online. 2013. Available online: http://www.nzbirdsonline.org.nz (accessed on 17 April 2020).
- Alexander, R.M. Allometry of the leg bones of moas (Dinornithes) and other birds. J. Zool. Lond. 1983, 200, 215–231. [Google Scholar] [CrossRef]
- Campbell, K.E., Jr.; Marcus, L. The relationship of hindlimb bone dimensions to body weight in birds. Nat. Hist. Mus. Los Angeles County Sci. Ser. 1992, 36, 395–412. [Google Scholar]





| A. Mean Measurement Values (mm) | |||||||
| Measurement | G. gallus | L. ocellata | A. cornuta | A. semipalmata | D. murrayi | D. planei | I. woodburnei |
| Wlst L | 12.47 | 14.32 | 16.79 | 20.50 | 55.90 | 67.65 | 51.32 |
| Wlst W | 4.81 | 5.52 | 6.34 | 7.92 | 27.63 | 34.43 | 28.87 |
| Tel.r L | 5.59 | 9.59 | 10.37 | 17.73 | N/A | N/A | N/A |
| Tel.r W | 4.02 | 5.45 | 8.13 | 11.93 | N/A | N/A | N/A |
| Tel.c L | 15.46 | 12.75 | 17.30 | 17.52 | 47.26 | 49.71 | 41.88 |
| Tel.c W | 12.91 | 13.40 | 18.43 | 22.18 | 41.14 | 40.61 | 30.52 |
| Opt L | 15.08 | 18.25 | 13.37 | 15.87 | 16.96 | 19.11 | 16.72 |
| Opt W | 5.98 | 7.81 | 4.67 | 4.85 | 6.75 | 5.96 | 8.02 |
| Tri.g L | 5.96 | 4.32 | 7.95 | 9.60 | 13.47 | 13.76 | 12.30 |
| Tri.g W | 4.83 | 3.76 | 3.03 | 3.32 | 9.56 | 9.20 | 9.18 |
| Cer L | 12.73 | 10.95 | 17.53 | 15.27 | 17.79 | 20.85 | 21.24 |
| Cer W | 10.71 | 9.91 | 13.63 | 17.55 | 37.87 | 45.99 | 33.21 |
| Rho L | 9.69 | 10.71 | 13.64 | 14.54 | 25.65 | 26.82 | 23.47 |
| Rho W | 7.66 | 9.51 | 12.39 | 10.03 | 18.19 | 18.02 | 13.83 |
| Tel.c TW | 21.07 | 22.25 | 28.21 | 31.16 | 67.34 | 72.83 | 57.52 |
| Meten TH | 15.43 | 16.26 | 20.34 | 22.04 | 38.85 | 40.68 | 34.56 |
| Med.Ob TW | 12.94 | 11.66 | 14.33 | 15.50 | 37.87 | 40.59 | 28.91 |
| Endo Vol (mm3) | 3733.84 | 4519.27 | 8031.85 | 10881.35 | 95577.71 | 122859.93 | 60289.34 |
| B. Mean Measurement Log Shape Ratios | |||||||
| Wlst L | 0.127 | 0.163 | 0.157 | 0.186 | 0.362 | 0.415 | 0.368 |
| Wlst W | −0.287 | −0.251 | −0.266 | −0.227 | 0.056 | 0.122 | 0.119 |
| Tel.r L | −0.221 | −0.011 | −0.052 | 0.123 | NA | NA | NA |
| Tel.r W | −0.365 | −0.257 | −0.158 | −0.049 | NA | NA | NA |
| Tel.c L | 0.220 | 0.113 | 0.170 | 0.118 | 0.289 | 0.281 | 0.280 |
| Tel.c W | 0.142 | 0.134 | 0.198 | 0.220 | 0.229 | 0.193 | 0.143 |
| Opt L | 0.210 | 0.268 | 0.058 | 0.075 | −0.156 | −0.134 | −0.119 |
| Opt W | −0.193 | −0.101 | −0.398 | −0.440 | −0.556 | −0.640 | −0.438 |
| Tri.g L | −0.194 | −0.358 | −0.167 | −0.143 | −0.256 | −0.276 | −0.252 |
| Tri.g W | −0.285 | −0.418 | −0.587 | −0.604 | −0.405 | −0.452 | −0.379 |
| Cer L | 0.136 | 0.046 | 0.176 | 0.058 | −0.135 | −0.096 | −0.015 |
| Cer W | 0.061 | 0.003 | 0.067 | 0.119 | 0.193 | 0.248 | 0.180 |
| Rho L | 0.017 | 0.037 | 0.067 | 0.037 | 0.024 | 0.013 | 0.029 |
| Rho W | −0.085 | −0.015 | 0.025 | −0.124 | −0.125 | −0.159 | −0.201 |
| Tel.c TW | 0.355 | 0.354 | 0.382 | 0.368 | 0.443 | 0.447 | 0.418 |
| Meten TH | 0.219 | 0.218 | 0.240 | 0.218 | 0.204 | 0.194 | 0.197 |
| Med.Ob TW | 0.143 | 0.074 | 0.088 | 0.065 | 0.193 | 0.193 | 0.119 |
| Module | C. Mean modular Surface Area Values (mm2) | ||||||
| Wlst | 58.50 | 65.71 | 90.65 | 117.40 | 1353.96 | 1851.49 | 1170.99 |
| Tel.r | 19.62 | 47.39 | 82.55 | 216.04 | NA | NA | NA |
| Tel.c | 144.52 | 133.10 | 249.41 | 297.48 | 1213.28 | 1297.48 | 852.94 |
| Opt | 78.90 | 118.28 | 52.33 | 71.57 | 139.37 | 164.03 | 145.83 |
| Tri.g F | 1.342 | 1.335 | 5.114 | 6.237 | 14.537 | 13.477 | 10.431 |
| Cer | 124.10 | 103.59 | 205.25 | 192.07 | 678.37 | 816.82 | 528.19 |
| Rho | 63.21 | 86.91 | 136.69 | 135.38 | 394.24 | 585.79 | 290.76 |
| Endo Surf (mm2) | 1544.33 | 1682.29 | 2404.61 | 2985.52 | 13199.82 | 15874.02 | 10200.52 |
| D. Mean Modular Surface Area Log Shape Ratios | |||||||
| Wlst | 0.176 | 0.136 | 0.079 | 0.078 | 0.655 | 0.720 | 0.708 |
| Tel.r | −0.299 | −0.006 | 0.039 | 0.343 | NA | NA | NA |
| Tel.c | 0.569 | 0.443 | 0.519 | 0.482 | 0.608 | 0.566 | 0.571 |
| Opt | 0.306 | 0.391 | −0.159 | −0.137 | −0.332 | −0.332 | −0.196 |
| Tri.g F | −1.464 | −1.556 | −1.169 | −1.197 | −1.314 | −1.418 | −1.342 |
| Cer | 0.502 | 0.334 | 0.434 | 0.292 | 0.355 | 0.365 | 0.363 |
| Rho | 0.210 | 0.258 | 0.258 | 0.140 | 0.119 | 0.221 | 0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handley, W.D.; Worthy, T.H. Endocranial Anatomy of the Giant Extinct Australian Mihirung Birds (Aves, Dromornithidae). Diversity 2021, 13, 124. https://doi.org/10.3390/d13030124
Handley WD, Worthy TH. Endocranial Anatomy of the Giant Extinct Australian Mihirung Birds (Aves, Dromornithidae). Diversity. 2021; 13(3):124. https://doi.org/10.3390/d13030124
Chicago/Turabian StyleHandley, Warren D., and Trevor H. Worthy. 2021. "Endocranial Anatomy of the Giant Extinct Australian Mihirung Birds (Aves, Dromornithidae)" Diversity 13, no. 3: 124. https://doi.org/10.3390/d13030124
APA StyleHandley, W. D., & Worthy, T. H. (2021). Endocranial Anatomy of the Giant Extinct Australian Mihirung Birds (Aves, Dromornithidae). Diversity, 13(3), 124. https://doi.org/10.3390/d13030124