Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine
Abstract
:1. Introduction
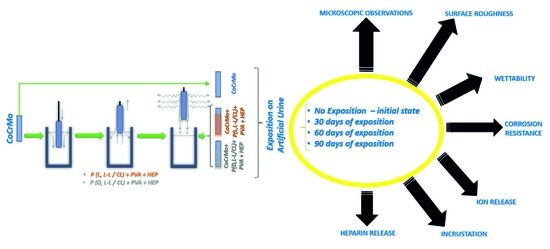
2. Materials and Methods
2.1. Surface Preparation
2.2. Microscopic Observations
2.3. Surface Roughness
2.4. Wettability Tests
2.5. Adhesion Test
2.6. Potentiodynamic Corrosion Test
2.7. Potentiostatic Corrosion Test
2.8. Investigation of the Mass Density of Ions Released into the Solution
2.9. Examination of Surface Degradation and Incrustation
2.10. Investigation of the Kinetics of the Heparin Release Process
2.11. Statistical Analysis
3. Results and Discussion
3.1. Results of Microscopic Observations
3.2. Surface Roughness Test Results
3.3. The Results of the Surface Wettability Tests
3.4. Results of Tests of Coating Adhesion to the Substrate
3.5. Results of Potentiodynamic Tests of Resistance to Pitting Corrosion
3.6. Results of Potentiostatic Tests of Resistance to Crevice Corrosion
3.7. The Results of the Analysis on the Mass Density of Ions Released into the Solution
3.8. The Results of the Surface Degradation and Incrustation Studies
3.9. The Results of the Studies on the Kinetics of the Heparin Release Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Türk, C.; Neisius, A.; Petřík, A.; Seitz, C.; Skolarikos, A.; Somani, B.; Thomas, K.; Gambaro, G.; Davis, N.F.; Donaldson, J.F.; et al. EAU Guidelines on Urolithiasis; Edn. Presented at the EAU Annual Congress Milan; EAU: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Wein, A.J.; Kavoussi, L.R.; Campbell, M.F.; Walsh, P.C. Campbell-Walsh Urology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- NFZ Online Data Base. Available online: https://statystyki.nfz.gov.pl/ (accessed on 3 March 2021).
- Mosayyebi, A.; Manes, C.; Carugo, D.; Somani, B.K. Advances in Ureteral Stent Design and Materials. Curr. Urol. Rep. 2018, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Różański, W.; Klimek, L.; Leńko, K.; Kliś, R. Zmiany zachodzące na powierzchni i w świetle cewnika podwójnie zgiętego wprowadzonego do dróg moczowych. Urol. Pol. 2005, 58, 3. [Google Scholar]
- Klimek, L.; Różański, W.; Jabłonowski, Z.; Sosnowski, M.; Kliś, R. Obserwacje zmian mikroskopowych cewników typu Double J w zależności od czasu utrzymywania ich w drogach moczowych. Inżynieria Biomater. 2005, 43–44, 36–39. [Google Scholar]
- Tunney, M.M.; Jones, D.S.; Gorman, S.P. Methacrylate polymers and copolymers as urinary tract biomaterials: Resistance to incrustation and microbial adhesion. Int. J. Pharm. 1997, 151, 121–126. [Google Scholar] [CrossRef]
- Denstedt, J.D.; Wollin, T.A.; Reid, G. Biomaterials used in urology: Current issues of biocompatibility, infection, and incrustation. J. Endourol. 1998, 12, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.; Elwood, C.N.; Choi, K.; Hendlin, K.; Monga, M.; Chew, B.H. Uropathogen Interaction With the Surface of Urological Stents Using Different Surface Properties. J. Urol. 2009, 182, 1194–1200. [Google Scholar] [CrossRef]
- Multanen, M.; Tammela, T.L.J.; Laurila, M.; Seppälä, J.; Välimaa, T.; Törmälä, P.; Talja, M. Biocompatibility, incrustation and biodegradation of ofloxacine and silver nitrate coated poly-L-lactic acid stents in rabbit urethra. Urol. Res. 2002, 30, 227–232. [Google Scholar] [CrossRef]
- Pedro, R.N.; Hendlin, K.; Kriedberg, C.; Monga, M. Wire-Based Ureteral Stents: Impact on Tensile Strength and Compression. Urology 2007, 70, 1057–1059. [Google Scholar] [CrossRef]
- Miyazaki, J.; Onozawa, M.; Takahashi, S.; Maekawa, Y.; Yasuda, M.; Wada, K.; Maeda, Y.; Masaki, T.; Yamaguchi, A.; Suzuki, M.; et al. The resonance® metallic ureteral stent in the treatment of malignant ureteral obstruction: A prospective observational study. BMC Urol. 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liatsikos, E.; Kallidonis, P.; Kyriazis, I.; Constantinidis, C.; Hendlin, K.; Stolzenburg, J.-U.; Karnabatidis, D.; Siablis, D. Ureteral Obstruction: Is the Full Metallic Double-Pigtail Stent the Way to Go? Eur. Urol. 2010, 57, 480–487. [Google Scholar] [CrossRef]
- Buchholz, N.; Hakenberg, O.; Masood, J.; Bach, C. Handbook of Urinary Stents. Basic Science and Clinical Applications; JP Medical Ltd.: London, UK, 2016. [Google Scholar]
- Frederick, L.; Ellimoottil, C.; Kadlec, A.; Shah, A.; Turk, T.; Schwartz, B.F. Cost Analysis of Metallic Stents for Chronic Ureteral Obstruction: A Multicenter Study. Urol Pract. 2016, 4, 1. [Google Scholar] [CrossRef]
- Baumgarten, A.S.; Hakky, T.S.; Carrion, R.E.; Lockhart, J.L.; Spiess, P.E. A Single-Institution Experience with Metallic Ureteral Stents: A Cost-Effective Method of Managing Deficiencies in Ureteral Drainage. Int. Braz. J. Urol. 2014, 40, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borin, J.F.; Melamud, O.; Clavman, R.V. Initial Experience with Full-Length Metal Stent to Relieve Malignant Ureteral Obstruction. J. Endourol. 2006, 5, 300–304. [Google Scholar] [CrossRef]
- Marciniak, J. Biomateriały; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2013. [Google Scholar]
- Park, J.; Kim, J.-K.; Patil, S.J.; Park, J.-K.; Park, S.; Lee, D.-W. A Wireless Pressure Sensor Integrated with a Biodegradable Polymer Stent for Biomedical Applications. Sensors 2016, 16, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinemann, S. Corrosion of surgical implants—In vivo in vitro test. In Advances in Biomaterials; Chirchester, W., Ed.; John Viley Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Lorenz, M.; Semlitsch, M.; Panic, B.; Weber, H.; Willert, H.G. Fatigue Strength of Cobalt-Base Alloys with High Corrosion Resistance for Artificial Hip Joints. Umschau 1978, 1, 241–250. [Google Scholar] [CrossRef]
- Marciniak, J.; Paszenda, Z.; Walke, W.; Kaczmarek, M.; Tyrlik-Held, J.; Kajzer, W. Stenty w Chirurgii Małoinwazyjnej. In Monografia; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2006. [Google Scholar]
- Liu, H.; Leng, Y.X.; Wan, G.; Huang, N. Corrosion susceptibility investigation of Ti-O film modified cobalt-chromium alloy (L-605) vascular stents by cyclic potentiodynamic polarization measurement. Surf. Coat. Tech. 2011, 206, 893–896. [Google Scholar] [CrossRef]
- Łaskawiec, J.; Michalik, R. Zagadnienia Teoretyczne i Aplikacyjne w Implantach; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2002. [Google Scholar]
- Nagai, A.; Tsutsumi, Y.; Suzuki, Y.; Katayama, K.; Hanawa, T.; Yamashita, K. Characterization of air-formed surface oxide film on a Co-Ni-Cr-Mo alloy (MP35N) and its change in Hanks’ solution. Appl. Surf. Sci. 2012, 258, 5490–5498. [Google Scholar] [CrossRef]
- Rylska, D.; Sokołowski, G.; Sokołowski, J.; Łukomska-Szymańska, M. Chemical passivation as a method of improving the electrochemical corrosion resistance of Co-Cr-based dental alloy. Acta Bioeng. Biomech. 2017, 19, 2. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Oyunbaatar, N.-E.; Lee, D.-H.; Patil, S.J.; Kim, E.-S.; Lee, D.-W. Biomechanical Characterization of Cardiomyocyte Using PDMS Pillar with Microgrooves. Sensors 2016, 16, 1258. [Google Scholar] [CrossRef] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Jelonek, K.; Kasperczyk, J. Polyesters and polyestercarbonates for controlled drug delivery Part I. Tailoring of the Drug Release. Polimery 2013, 58, 654–662. [Google Scholar] [CrossRef]
- Jelonek, K.; Kasperczyk, J. Polyesters and polyester carbonates for controlled drug delivery. Polimery 2013, 58, 858–863. [Google Scholar] [CrossRef]
- Albertsso, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. Adv. Polym. Sci. 2002, 157, 1–40. [Google Scholar]
- Jaworska, J.; Jelonek, K.; Jaworska-Kik, M.; Musiał-Kulik, M.; Marcinkowski, A.; Szewczenko, J.; Kajzer, W.; Pastusiak, M.; Kasperczyk, J. Development of antibacterial, ciprofloxacin-eluting biodegradable coatings on Ti6Al7Nb implants to prevent peri-implant infections. J. Biomed. Mater. Res. Part A 2020, 108, 1006–1015. [Google Scholar] [CrossRef]
- Bose, S.; Keller, S.S.; Alstrøm, T.S.; Boisen, A.; Almdal, K. Process Optimization of Ultrasonic Spray Coating of Polymer Films. Langmuir 2013, 29, 6911–6919. [Google Scholar] [CrossRef]
- Pieccka, S.; Paluch, D.; Staniszewska-Kuś, J.; Solski, L.; Lewandowska-Szumiet, M. Badania Biozgodności Materiałów Implantacyjnych. In Biomateriały Pod Red; Błażewicz, S., Stoch, L., Eds.; Akademicka Oficyna Wydawnicza: Warszawa, Poland, 2003; pp. 425–527. [Google Scholar]
- Wierzchoń, T.; Czarnowska, E.; Krupa, D. Inżyniera Powierzchni w Wytwarzaniu Biomateriałów Tytanowch; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2004. [Google Scholar]
- Wannerberg, A. The role of surface roughness for implant incorporation in bone. Cells Mater. 1999, 9, 1. [Google Scholar]
- Standford, C.M.; Keller, C.J.; Solursh, M. Bone cell expression on titanium surfaces is altered by serilization treatments. J. Dent. Res. 1994, 73, 1061. [Google Scholar] [CrossRef] [PubMed]
- Weroński, A.; Surowska, B.; Cieśla, M. Struktura i właściwości stopu kobaltu na endoprotezy. Inżynieria Mater. 1990, 5, 111–114. [Google Scholar]
- Błażewicz, S.; Stoch, L. Biocybernetyka i Inżynieria Biomedyczna 2000; Tom 4, Biomateriały; Akademicka Oficyna Wydawnicza EXIT: Warszawa, Poland, 2017. [Google Scholar]
- Standard: ASTM F:1828-97 (Reapproved 2014) Standard Specification for Ureteral Stents; ASTM International: West Conshohocken, PA, USA, 2014.
- Standard: PN-EN ISO 1302:2004-Specyfikacje Geometrii Wyrobów (GPS)-Oznaczanie Struktury Geometrycznej Powierzchni w Dokumentacji Technicznej Wyrobu; Polish Committee for Standardization: Warsaw, Poland, 2004.
- Standard: ASTM F746-04 (2009)-Standard Test Method for Pitting or Vrevice Corrosion of Metallic Surgical Implant Materials; ASTM International: West Conshohocken, PA, USA, 2009.
- Xiaoyi, X.; Qingbiao, Y.; Yongzhi, W.; Haijun, Y.; Xuesi, C.; Xiabin, J. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar]
- Warttinger, U.; Giese, C.; Krämer, R. Comparison of Heparin Red, Azure A and Toluidine Blue assays for direct quantification of heparins in human plasma. arXiv 2017, arXiv:1712.03377. [Google Scholar]
- Szewczenko, J.; Kajzer, W.; Grygiel-Rpadelok, M.; Jaworska, J.; Jelonek, K.; Nowińska, K.; Gawliczek, M.; Libera, M.; Marcinkowski, A.; Kasperczyk, J. Corrosion resistance of PLGA-coated biomaterials. Acta Bioeng. Biomech. 2017, 19, 173–179. [Google Scholar] [PubMed]
- Szewczenko, J.; Kajzer, W.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Antonowicz, M.; Nowińska, K.; Jaworska, J.; Jelonek, K.; Kasperczyk, J. Biodegradable polimer coatings on Ti6Al7Nb alloy. Acta Bioeng. Biomech. 2019, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kajzer, W.; Jaworska, J.; Jelonek, K.; Szewczenko, J.; Kajzer, A.; Nowińska, K.; Hercog, A.; Kasperczyk, J. Corrosion resistance of Ti6Al4V alloy coated with caprolactone-based biodegradable polymeric coatings. Eksploat. Niezawodn. 2018, 20, 30–38. [Google Scholar] [CrossRef]
- Szewczenko, J.; Kajzer, W.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Major, R.; Simka, W.; Jaworska, J.; Jelonek, K.; Karpeta-Jarząbek, P.; et al. Adhesion of Poly(lactide-glycolide) coating (PLGA) on the Ti6Al7Nb alloy substrate. In Advences in Intelligent Systems and Computing, Proceedings of the International Conference, ITIB 2019, Kamień Śląski, Poland, 18–20 June 2019; Pietka, E., Badura, P., Kawa, J., Wieclawek, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 578–589. [Google Scholar]
- Kajzer, W.; Szewczenko, J.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Antonowicz, M.; Jaworska, J.; Jelonek, K.; Orchel, A.; Nowińska, K.; et al. Electrochemical and biological performance of biodegradable polimer coatings on Ti6Al7Nb alloy. Materials 2020, 13, 1758. [Google Scholar] [CrossRef] [Green Version]
- Szewczenko, J.; Kajzer, W.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Antonowicz, M.; Jaworska, J.; Jelonek, K.; Marcinkowski, A.; Kasperczyk, J. Surface modification of titanium 6-aluminum 7-niobium alloy with biodegradable polimer coatings. Materialwiss. Werkstofftech. 2020, 51, 613–623. [Google Scholar] [CrossRef]
- Kajzer, W.; Szewczenko, J.; Kajzer, A.; Basiaga, M.; Jaworska, J.; Jelonek, K.; Hercog, A.; Kaczmarek, M.; Marcinkowski, A.; Kasperczyk, J. The effect of electron beam sterilization of the physical propoerties of the bioresorbable polymer coatings on the titanium 6-aluminum 4-vanadium substrate. Materialwiss. Werkstofftech. 2020, 51, 631–644. [Google Scholar] [CrossRef]
- Szewczenko, J.; Kajzer, W.; Kajzer, A.; Basiaga, M.; Kaczmarek, M.; Major, R.; Jaworska, J.; Jelonek, K.; Karpeta-Jarząbek, P.; Jaworska-Kik, M.; et al. Influence of surface modification of Ti6Al7Nb alloy on adhesion of poly(lactide-co-glycolide) coating (PLGA). Colloids Surf. B Biointerfaces 2020, 196, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Vert, G.M. Structure-property relationship in the case of the degradation of massive poly(-hydroxyacids) in aqueous media—Part 1: Poly(dl-lactic acid). J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- Saad, B.; Suter, U.W. Biodegradable polymeric materials. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; p. 551. [Google Scholar]
- Garcia-Lalcon, C.M.; Gil-Lopez, T.; Verdu-Vazquez, A.; Mirza-Rosca, J.C. Electrochemical characterization of some cobalt base alloys in Ringer solution. Mater. Chem. Phys. 2021, 260, 124164. [Google Scholar] [CrossRef]
- Gemmana, H.; Berges, C.; Naranjo, J.A.; Garrcia, C.; Garrido, I. Mechanical performance, corrosion and tribological evaluation of CoCrMo alloy processed by MIM for biomedical applications. J. Mech. Behav. Biomed. 2020, 105, 103706. [Google Scholar]














| Sample | Ra, µm |
|---|---|
| CoCrMo | 0.11 ± 0.02 |
| CoCrMo+P(L,L-L/CL) + PVA + HEP | 0.12 ± 0.02 |
| CoCrMo+P(D,L-L/CL) + PVA + HEP | 0.12 ± 0.02 |
| Sample | Critical Force Fn (N) after Exposition Time | |||
|---|---|---|---|---|
| 0 Days | 30 Days | 60 Days | 90 Days | |
| CoCrMo—CoCrMo + P(L,L-L/CL) | 4.74 | 7.85 | 0.03 | 0.03 |
| CoCrMo—CoCrMo + P(D,L-L/CL) | 5.92 | 6.94 | 2.28 | 1.50 |
| Samples | Exposition Time, Days | Ecorr, mV | SD | Etr, mV | SD | Rp, MΩ⋅cm2 | SD |
|---|---|---|---|---|---|---|---|
| CoCrMo | 0 | −72 | 15 | 790 | 7 | 2.0 | 0.3 |
| 30 | −151 | 56 | 775 | 5 | 1.3 | 0.8 | |
| 60 | −175 | 100 | 781 | 4 | 1.0 | 0.5 | |
| 90 | −101 | 12 | 770 | 1 | 2.1 | 0.3 | |
| CoCrMo+P(L,L-L/CL)+PVA+HEP | 0 | +1 | 10 | 964 | 19 | 6.3 | 3.4 |
| 30 | −145 | 49 | 858 | 37 | 2.0 | 0.2 | |
| 60 | −116 | 80 | 828 | 11 | 2.2 | 0.6 | |
| 90 | −68 | 50 | 828 | 17 | 2.0 | 0.4 | |
| CoCrMo+P(D,L-L/CL)+PVA+HEP | 0 | −59 | 57 | 955 | 7 | 5.8 | 1.3 |
| 30 | −84 | 9 | 778 | 1 | 1.6 | 0.1 | |
| 60 | −147 | 87 | 780 | 2 | 1.4 | 0.6 | |
| 90 | −50 | 37 | 783 | 2 | 0.8 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajzer, W.; Szewczenko, J.; Kajzer, A.; Basiaga, M.; Jaworska, J.; Jelonek, K.; Nowińska, K.; Kaczmarek, M.; Orłowska, A. Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine. Materials 2021, 14, 2551. https://doi.org/10.3390/ma14102551
Kajzer W, Szewczenko J, Kajzer A, Basiaga M, Jaworska J, Jelonek K, Nowińska K, Kaczmarek M, Orłowska A. Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine. Materials. 2021; 14(10):2551. https://doi.org/10.3390/ma14102551
Chicago/Turabian StyleKajzer, Wojciech, Janusz Szewczenko, Anita Kajzer, Marcin Basiaga, Joanna Jaworska, Katarzyna Jelonek, Katarzyna Nowińska, Marcin Kaczmarek, and Ada Orłowska. 2021. "Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine" Materials 14, no. 10: 2551. https://doi.org/10.3390/ma14102551
APA StyleKajzer, W., Szewczenko, J., Kajzer, A., Basiaga, M., Jaworska, J., Jelonek, K., Nowińska, K., Kaczmarek, M., & Orłowska, A. (2021). Physical Properties of Electropolished CoCrMo Alloy Coated with Biodegradable Polymeric Coatings Releasing Heparin after Prolonged Exposure to Artificial Urine. Materials, 14(10), 2551. https://doi.org/10.3390/ma14102551