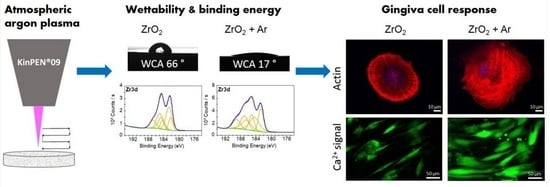
Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zirconia Specimens and Argon (Ar) Plasma Activation
2.2. Chemical Surface Properties
2.3. Gingival Cell Culture and Characterization
2.4. Cell Morphology and Actin Cytoskeleton Organization
2.5. Cell Spreading
2.6. Calcium Ion (Ca2+) Mobilization
2.7. Statistical Analysis
3. Results
3.1. Characterization of Chemical Surface Properties
3.2. Characterization of Human Gingival Fibroblasts (HGF-1)
3.2.1. Growth over Time
3.2.2. Expression of Adhesion Receptors
3.2.3. Expression of Adenosine 5′-Triphosphate (ATP) Receptors
3.3. Impact of Ar Plasma Activation on First Cell Response after 2 h
3.4. Impact of Ar Plasma Activation on Cell Phenotype after 24 h
3.5. Impact of Ar Plasma Activation on Calcium Ion (Ca2+) Mobilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davoudi, A.; Salimian, K.; Tabesh, M.; Attar, B.M.; Golrokhian, M.; Bigdelou, M. Relation of CAD/CAM zirconia dental implant abutments with periodontal health and final aesthetic aspects; A systematic review. J. Clin. Exp. Dent. 2023, 15, e64–e70. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.H.D.H.; Barros, A.W.P.; Oliveira-Neto, O.B.; de Lima, F.J.C.; Carvalho, A.A.T.; Leão, J.C. Do zirconia dental implants present better clinical results than titanium dental implants? A systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101324. [Google Scholar] [CrossRef] [PubMed]
- Rohr, N.; Fricke, K.; Bergemann, C.; Nebe, J.B.; Fischer, J. Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years. J. Clin. Med. 2020, 9, 2776. [Google Scholar] [CrossRef] [PubMed]
- Naveau, A.; Rignon-Bret, C.; Wulfman, C. Zirconia abutments in the anterior region: A systematic review of mechanical and esthetic outcomes. J. Prosthet. Dent. 2019, 121, 775–781.e1. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M. Future prospects of zirconia for oral implants—A review. Dent Mater. J. 2020, 39, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29, 135–153. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P. Biologic width around implants. An evidence-based review. Stomatologija 2008, 10, 27–35. [Google Scholar]
- Rohr, N.; Balmer, M.; Jung, R.E.; Kohal, R.J.; Spies, B.C.; Hämmerle, C.H.F.; Fischer, J. Influence of zirconia implant surface topography on first bone implant contact within a prospective cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 593–599. [Google Scholar] [CrossRef]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface. Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.-J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implant. Res. 2008, 19, 635–641. [Google Scholar] [CrossRef]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000, 2000, 28–55. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.; Magini, R.S.; Miranda, G.; Silva, F.S.; da Mata, A.D.S.P.; Caramês, J.M.M. Hard and Soft Tissue Cell Behavior on Polyetheretherketone, Zirconia, and Titanium Implant Materials. Int. J. Oral Maxillofac. Implant. 2019, 34, 39–46. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 2—Review focusing on clinical knowledge of different surfaces. Int. J. Prosthodont. 2004, 17, 544–564. [Google Scholar]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 55–67. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, K.; Odén, A.; Wennerberg, A.; Hultenby, K.; Arvidson, K. The influence of surface topography of ceramic abutments on the attachment and proliferation of human oral fibroblasts. Biomaterials 2005, 26, 373–381. [Google Scholar] [CrossRef]
- Staehlke, S.; Springer, A.; Freitag, T.; Brief, J.; Nebe, J.B. The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles. Dent. J. 2020, 8, 127. [Google Scholar] [CrossRef]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, J.B. Laser structured dental zirconium for soft tissue cell occupa-tion—Importance of wettability modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric pressure plasma jet for medical therapy: Plasma parameters and risk estimation. Contrib Plasma Physics. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Iaculli, F.; Piattelli, A.; Quaranta, A. The emerging role of cold atmospheric plasma in implantology: A review of the literature. Nanomaterials 2020, 10, 1505. [Google Scholar] [CrossRef]
- Tabares, F.L.; Junkar, I. Cold Plasma Systems and their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Rolauffs, B.; Adolfsson, E.; Altmann, B. Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma. Sci. Rep. 2021, 11, 17302. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas. Res. 2013, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Gruening, M.; Neuber, S.; Nestler, P.; Lehnfeld, J.; Dubs, M.; Fricke, K.; Schnabelrauch, M.; Helm, C.A.; Müller, R.; Staehlke, S.; et al. Enhancement of Intracellular Calcium Ion Mobilization by Moderately but Not Highly Positive Material Surface Charges. Front. Bioeng. Biotechnol. 2020, 8, 1016. [Google Scholar] [CrossRef]
- Moerke, C.; Rebl, H.; Finke, B.; Dubs, M.; Nestler, P.; Airoudj, A.; Roucoules, V.; Schnabelrauch, M.; Körtge, A.; Anselme, K.; et al. Abrogated cell contact guidance on amino-functionalized microgrooves. ACS Appl. Mater. Interfaces. 2017, 9, 10461–10471. [Google Scholar] [CrossRef]
- Finke, B.; Rebl, H.; Hempel, F.; Schäfer, J.; Liefeith, K.; Weltmann, K.-D.; Nebe, J.B. Ageing of plasma-polymerised allylamine nanofilms and the maintenance of their cell adhesion capacity. Langmuir 2014, 30, 13914–13924. [Google Scholar] [CrossRef]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef] [Green Version]
- Bershadsky, A.D.; Kozlov, M.M. Crawling cell locomotion revisited. Proc. Natl. Acad. Sci. USA 2011, 108, 20275e6. [Google Scholar] [CrossRef] [Green Version]
- Bootman, M.D.; Berridge, M.J.; Roderick, H.L. Calcium signalling: More messengers, more channels, more complexity. Curr. Biol. 2002, 12, 563e5. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, R.; Maselli, A.; Thomson, S.A.; Lim, R.W.; Stokes, J.V.; Fechheimer, M. Calcium regulation of actin crosslinking is important for function of the actin cytoskeleton in dictyostelium. J. Cell Sci. 2003, 116 Pt 1, 187e96. [Google Scholar] [CrossRef] [Green Version]
- Canullo, L.; Genova, T.; Gross Trujillo, E.; Pradies, G.; Petrillo, S.; Muzzi, M.; Carossa, S.; Mussano, F. Fibroblast interaction with different abutment surfaces: In vitro study. Int. J. Mol. Sci. 2020, 21, 1919. [Google Scholar] [CrossRef] [Green Version]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Heuberger, R.; Hanisch, O.; Schwarz, F.; Precht, C. Influence of UV Irradiation and Cold Atmospheric Pressure Plasma on Zirconia Surfaces: An In Vitro Study. Int. J. Oral Maxillofac. Implants. 2019, 34, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667e81. [Google Scholar] [CrossRef] [PubMed]
- Hoentsch, M.; von Woedtke, T.; Weltmann, K.-D.; Nebe, J.B. Time-dependent effects of low-temperature atmospher-ic-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J. Phys. D Appl. Phys. 2012, 45, 025206. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces: An In Vitro Comparative Study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef]
- Danna, N.R.; Beutel, B.G.; Tovar, N.; Witek, L.; Marin, C.; Bonfante, E.A.; Granato, R.; Suzuki, M.; Coelho, P.G. Assessment of Atmospheric Pressure Plasma Treatment for Implant Osseointegration. BioMed Res. Int. 2015, 2015, 761718. [Google Scholar] [CrossRef]
- Nebe, J.B.; Rebl, H.; Schlosser, M.; Staehlke, S.; Gruening, M.; Weltmann, K.D.; Walschus, U.; Finke, B. Plasma Polymerized Allylamine-The Unique Cell-Attractive Nanolayer for Dental Implant Materials. Polymers 2019, 11, 1004. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [Green Version]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [Green Version]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Choi, S.H.; Ryu, J.H.; Kwon, J.S.; Kim, J.E.; Cha, J.Y.; Lee, K.J.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Effect of wet storage on the bioactivity of ultraviolet light- and non-thermal atmospheric pressure plasma-treated titanium and zirconia implant surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110049. [Google Scholar] [CrossRef]
- Komasa, S.; Kusumoto, T.; Hayashi, R.; Takao, S.; Li, M.; Yan, S.; Zeng, Y.; Yang, Y.; Hu, H.; Kobayashi, Y.; et al. Effect of Argon-Based Atmospheric Pressure Plasma Treatment on Hard Tissue Formation on Titanium Surface. Int. J. Mol. Sci. 2021, 22, 7617. [Google Scholar] [CrossRef]
- Garcia, B.; Camacho, F.; Peñarrocha, D.; Tallarico, M.; Perez, S.; Canullo, L. Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: A randomized controlled histologic study. Clin. Oral Implant. Res. 2017, 28, 1269–1277. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, K.; Kokubun, K.; Sasaki, H.; Yoshinari, M. Change in surface properties of zirconia and initial attachment of osteoblastlike cells with hydrophilic treatment. Dent. Mater. J. 2012, 31, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Chambers, J.J.; Parsons, G.N. Yttrium silicate formation on silicon: Effect of silicon preoxidation and nitridation on interface reaction kinetics. Appl. Phys. Lett. 2000, 77, 2385–2387. [Google Scholar] [CrossRef]
- Guittet, M.J.; Crocombette, J.P.; Gautier-Soyer, M. Bonding and XPS chemical shifts in ZrSiO4 versus SiO2 and ZrO2: Charge transfer and electrostatic effects. Phys. Rev. B 2001, 63, 125117. [Google Scholar] [CrossRef]
- Foest, R.; Schmidt, M.; Becker, K. Microplasmas, a New World of low-temperature plasmas. Int. J. Mass Spectrom. 2005, 248, 87–102. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Cell-adhesion to plasma-treated polymer surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Parizek, M.; Slepickova Kasalkova, N.; Bacakova, L.; Svindrych, Z.; Slepicka, P.; Bacakova, M.; Lisa, V.; Svorcik, V. Adhesion, growth, and maturation of vascular smooth muscle cells on low-density polyethylene grafted with bioactive substances. BioMed Res. Int. 2013, 2013, 371430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant. Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157e73. [Google Scholar] [CrossRef] [Green Version]
- Mooney, D.J.; Langer, R.; Ingber, D.E. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J. Cell. Sci. 1995, 108, 2311e20. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Dimchev, G.; Amiri, B.; Humphries, A.C.; Schaks, M.; Dimchev, V.; Stradal, T.E.B.; Faix, J.; Krause, M.; Way, M.; Falcke, M.; et al. Lamellipodin tunes cell migration by stabilizing protrusions and promoting adhesion formation. J. Cell Sci. 2020, 133, jcs239020. [Google Scholar] [CrossRef]
- Boraschi-Diaz, I.; Mort, J.S.; Brömme, D.; Senis, Y.A.; Mazharian, A.; Komarova, S.V. Collagen type I degradation fragments act through the collagen receptor LAIR-1 to provide a negative feedback for osteoclast formation. Bone 2018, 117, 23–30. [Google Scholar] [CrossRef]
- Nebe, B.; Rychly, J.; Knopp, A.; Bohn, W. Mechanical induction of b1-integrinmediated calcium signaling in a hepatocyte cell line. Exp. Cell Res. 1995, 218, 479–484. [Google Scholar] [CrossRef]
- Prasad, M.; Fearon, I.M.; Zhang, M.; Laing, M.; Vollmer, C.; Nurse, C.A. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: Role in chemosensory signalling. J. Physiol. 2001, 537 Pt 3, 667–677. [Google Scholar] [CrossRef]
- Florenzano, F.; Viscomi, M.T.; Cavaliere, F.; Volonté, C.; Molinari, M. Cerebellar lesion up-regulates P2X1 and P2X2 purinergic receptors in precerebellar nuclei. Neuroscience 2002, 115, 425–434. [Google Scholar] [CrossRef]
- Diener, A.; Nebe, B.; Lüthen, F.; Becker, P.; Beck, U.; Neumann, H.G.; Rychly, J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials 2005, 26, 383–392. [Google Scholar] [CrossRef]









| Element | Peak Area | ZrO2 | ZrO2 + Ar |
|---|---|---|---|
| Si2p | absolute (cps) | 4704 | 3021 |
| relative (%) | 1.59 | 0.62 | |
| Zr3d | absolute (cps) | 71,442 | 185,766 |
| relative (%) | 24.08 | 37.94 | |
| Y3d | absolute (cps) | 4019 | 11,071 |
| relative (%) | 1.35 | 2.26 | |
| O1s | absolute (cps) | 111,786 | 255,777 |
| relative (%) | 37.67 | 52.24 | |
| C1s | absolute (cps) | 104,768 | 34,021 |
| relative (%) | 35.31 | 6.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staehlke, S.; Brief, J.; Senz, V.; Eickner, T.; Nebe, J.B. Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials 2023, 16, 4203. https://doi.org/10.3390/ma16124203
Staehlke S, Brief J, Senz V, Eickner T, Nebe JB. Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials. 2023; 16(12):4203. https://doi.org/10.3390/ma16124203
Chicago/Turabian StyleStaehlke, Susanne, Jakob Brief, Volkmar Senz, Thomas Eickner, and J. Barbara Nebe. 2023. "Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation" Materials 16, no. 12: 4203. https://doi.org/10.3390/ma16124203
APA StyleStaehlke, S., Brief, J., Senz, V., Eickner, T., & Nebe, J. B. (2023). Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials, 16(12), 4203. https://doi.org/10.3390/ma16124203