Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Results
2.1.1. Structure and Morphology of CuMgFe-xLDH and CuMgFe-xLDO
2.1.2. Magnetic Behavior of CuMgFe-xLDO Catalysts
2.1.3. H2-TPR of CuMgFe-xLDO Catalysts
2.1.4. XPS Analysis of CuMgFe-xLDO Catalysts
2.1.5. Basicity of Reduced Catalysts
2.1.6. H2-TPD of Reduced Catalysts
2.2. Hydrogenolysis of Glycerol
2.2.1. Effect of (Cu + Mg)/Fe Molar Ratio on Catalytic Performance of Reduced CuMgFe-xLDO Catalysts
2.2.2. Hydrogenolysis of Glycerol on Reduced CuMgFe-4LDO Catalyst at Different Temperatures
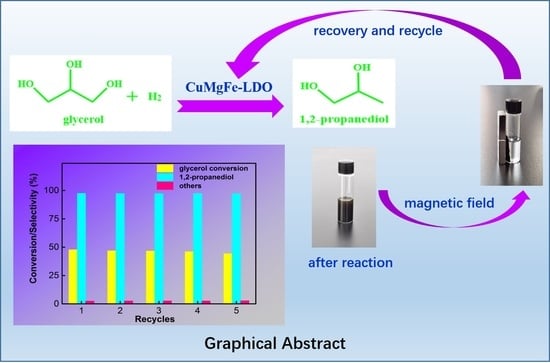
2.2.3. Recycled Usage of Reduced CuMgFe-4LDO Catalyst
3. Materials and Methods
3.1. Preparation of CuMgFe-Mixed Oxides Catalysts
3.2. Characterization of Precursors and Catalysts
3.3. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Dalil, M.; Edake, M.; Sudeau, C.; Dubois, J.L.; Patience, G.S. Coke promoters improve acrolein selectivity in the gas-phase dehydration of glycerol to acrolein. Appl. Catal. A Gen. 2016, 522, 80–89. [Google Scholar] [CrossRef]
- Yun, D.; Yun, Y.S.; Kim, T.Y.; Park, H.; Lee, J.M.; Han, J.W.; Yi, J. Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate. J. Catal. 2016, 341, 33–43. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jiang, H.; Chen, C.; Chen, R.; Xing, W. Solvent effect on hydrogenolysis of glycerol to 1,2-propanediol over Cu–ZnO catalyst. Chem. Eng. J. 2015, 264, 344–350. [Google Scholar] [CrossRef]
- Dam, J.T.; Hanefeld, U. Renewable chemicals: Dehydroxylation of glycerol and polyols. ChemSusChem 2011, 4, 1017–1034. [Google Scholar]
- Wang, Y.; Zhou, J.; Guo, X. Catalytic hydrogenolysis of glycerol to propanediols: A review. RSC Adv. 2015, 5, 74611–74628. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B Environ. 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Mane, R.; Patil, S.; Shirai, M.; Rayalu, S.; Rode, C. Influence of carbon based supports on selectivity behavior of diols and pro panol in Ru catalyzed glycerol hydrogenolysis. Appl. Catal. B Environ. 2017, 204, 134–146. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N.; Sai Prasad, P.S.; Lingaiah, N. Surface and structural proper ties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A Gen. 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Wang, S.; Ma, X. The effect of metal properties on the reaction routes of glycerol hydrogenolysis over platinum and ruthenium catalysts. Catal. Today 2017, 298, 2–8. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Shinmi, Y.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Modification of Rh/SiO2 catalyst for the hydrogenolysis of glycerol in water. Appl. Catal. B Environ. 2010, 94, 318–326. [Google Scholar] [CrossRef]
- Rodrigues, R.; Isoda, N.; Gonçalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Carvalho, W.A. Effect of niobia and alumina as support for Pt catalysts in the hydrogenolysis of glycerol. Chem. Eng. J. 2012, 198–199, 457–467. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. Glycerol hydrogenolysis to 1,3-propanediol on tungstate/zirconia-supported platinum: Hydrogen spillover facilitated by Pt(111) Formation. ChemCatChem 2016, 8, 3663–3671. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Xiu, J.; Wang, Y.; Williams, C.T.; Liang, C. Synergetic effect between Cu° and Cu+ in the Cu-Cr catalysts for hydrogenolysis of glycerol. Catal. Today 2014, 234, 200–207. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, H.; Wang, A.; Shen, L.; Yu, L.; Jiang, T. Gas phase hydrogenolysis of glycerol catalyzed by Cu/ZnO/MOx (MOx = Al2O3, TiO2, and ZrO2) catalysts. Chem. Eng. J. 2011, 168, 403–412. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, H.; Liu, Y.; Chen, R. Role of initial water content in glycerol hydrogenolysis to 1,2-propanediol over Cu–ZnO catalyst. Reac. Kinet. Mech. Cat. 2017, 122, 1129–1143. [Google Scholar] [CrossRef]
- Vila, F.; López Granados, M.; Ojeda, M.; Fierro, J.L.G.; Mariscal, R. Glycerol hydrogenolysis to 1,2-propanediol with Cu/γ-Al2O3: Effect of the activation process. Catal. Today 2012, 187, 122–128. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H.; Cui, F.; Zuo, J.; Chen, J.; Xia, C. Effects of the precipitation agents and rare earth additives on the structure and catalytic performance in glycerol hydrogenolysis of Cu/SiO2 catalysts prepared by precipitation-gel method. Catal. Today 2014, 234, 223–232. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Pandhare, N.N.; Pudi, S.M.; Biswas, P.; Sinha, S. Selective hydrogenolysis of glycerol to 1,2-propanediol over highly active and stable Cu/MgO catalyst in the vapor phase. Org. Process Res. Dev. 2016, 20, 1059–1067. [Google Scholar] [CrossRef]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Xia, S.; Zheng, L.; Nie, R.; Chen, P.; Lou, H.; Hou, Z. Trivalent metal ions M3+ in M0.02Cu0.4Mg5.6Al1.98(OH)16CO3 layered double hydroxide as catalyst precursors for the hydrogenolysis of glycerol. Chin. J. Catal. 2013, 34, 986–992. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A Gen. 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Mondal, S.; Janardhan, R.; Lal Meena, M.; Biswas, P. Highly active Cu-Zn-Mg-Al-O catalyst derived from layered double hydroxides (LDHs) precursor for selective hydrogenolysis of glycerol to 1,2-propanediol. J. Environ. Chem. Eng. 2017, 5, 5695–5706. [Google Scholar] [CrossRef]
- Geng, G.; Wei, R.; Liang, T.; Zhou, M.; Xiao, G. Hydrogenolysis of glycerol to propanediols on Cu–Ca–Al hydrotalcites derived catalysts. Reac. Kinet. Mech. Catal. 2016, 117, 239–251. [Google Scholar] [CrossRef]
- Fan, G.; Li, F. Effect of sodium borohydride on growth process of controlled flower-like nanostructured Cu2O/CuO films and their hydrophobic property. Chem. Eng. J. 2011, 167, 388–396. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Srinivasan, M. Electrospun CuFe2O4 nanotubes as anodes for high-performance lithium-ion batteries. J. Energy Chem. 2014, 23, 301–307. [Google Scholar] [CrossRef]
- Shafi, K.V.P.M.; Gedanken, A.; Prozorov, R.; Balogh, J. Sonochemical preparation and size-dependent properties of nanostructured CoFe2O4 particles. Chem. Mater. 1998, 10, 3445–3450. [Google Scholar] [CrossRef]
- Xiao, Z.; Jin, S.; Wang, X.; Li, W.; Wang, J.; Liang, C. Preparation, structure and catalytic properties of magnetically separable Cu-Fe catalysts for glycerol hydrogenolysis. J. Mater. Chem. 2012, 22, 16598–16605. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, Y.; Liu, J.; Huang, Q.; He, S.; Li, C.; Zhao, J.; Wei, M. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides. Catal. Sci. Technol. 2013, 3, 1324–1332. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Remiro, A.; Vicente, J.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Stability of CuZnOAl2O3/HZSM-5 and CuFe2O4/HZSM-5 catalysts in dimethyl ether steam reforming operating in reaction–regeneration cycles. Fuel Process. Technol. 2014, 126, 145–154. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Yin, L. Effect of various precipitants on activity and thermal stability of CuFe2O4 water-gas shift catalysts. J. Fuel Chem. Technol. 2014, 42, 1087–1092. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, L.; You, T.; Li, C.; Shan, H. Effect of sulfation on the performance of Fe2O3/Al2O3 catalyst in catalytic dehydrogenation of propane to propylene. Chem. Eng. J. 2014, 244, 145–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In situ DRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Shi, X.; Chu, B.; Wang, F.; Wei, X.; Teng, L.; Fan, M.; Li, B.; Dong, L.; Dong, L. Mn-modified CuO, CuFe2O4 and γ-Fe2O3 three-phase strong synergistic coexistence catalyst system for NO reduction by CO with wider active window. ACS Appl. Mater. Interfaces 2018, 10, 40509–40522. [Google Scholar] [CrossRef]
- Faheem, M.; Jiang, X.; Wang, L.; Shen, J. Synthesis of Cu2O–CuFe2O4 microparticles from Fenton sludge and its application in the Fenton process: The key role of Cu2O in the catalytic degradation of phenol. RSC Adv. 2018, 8, 5740–5748. [Google Scholar] [CrossRef] [Green Version]
- Karim, K.M.R.; Tarek, M.; Ong, H.R.; Abdullah, H.; Yousuf, A.; Cheng, C.K.; Khan, M.M.R. Photoelectrocatalytic reduction of carbon dioxide to methanol using CuFe2O4 modified with graphene oxide under visible light irradiation. Ind. Eng. Chem. Res. 2019, 58, 563–572. [Google Scholar] [CrossRef]
- Dubecký, F.; Kindl, D.; Hubík, P.; Mičušík, M.; Dubecký, M.; Boháček, P.; Vanko, G.; Gombia, E.; Nečas, V.; Mudroň, J. A comparative study of Mg and Pt contacts on semi-insulating GaAs: Electrical and XPS characterization. Appl. Surf. Sci. 2017, 395, 131–135. [Google Scholar] [CrossRef]
- Li, W.H.; Sun, Y.H. Surface properties and CO adsorption on zirconia polymorphs. J. Mol. Catal. A Chem. 2005, 227, 119–124. [Google Scholar]
- Coleman, L.J.I.; Epling, W.; Hudgins, P.R.; Croiset, E. Ni/Mg–Al mixed oxide catalyst for the steam reforming of ethanol. Appl. Catal. A Gen. 2009, 363, 52–63. [Google Scholar] [CrossRef]
- Yu, X.; Wang, N.; Chu, W.; Liu, M. Carbon dioxide reforming of methane for syngas production over La-promoted NiMgAl catalysts derived from hydrotalcites. Chem. Eng. J. 2012, 209, 623–632. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, X.; Chen, D.; Xiao, W. An effective iron catalyst supported on mixed MgO-Al2O3 for Fischer-Tropsch synthesis to olefins. Ind. Eng. Chem. Res. 2020, 59, 11462–11474. [Google Scholar] [CrossRef]
- Balaraju, M.; Jagadeeswaraiah, K.; Sai Prasad, P.S.; Lingaiah, N. Catalytic hydrogenolysis of biodiesel derived glycerol to 1,2-propanediol over Cu–MgO catalysts. Catal. Sci. Technol. 2012, 2, 1967–1976. [Google Scholar] [CrossRef]
- Li, S.; Lv, S.; Zhang, Y.; Li, J.; Liu, Z.; Wang, L. Syngas-derived olefins over iron-based catalysts: Effects of basic properties of MgO nanocrystals. J. Fuel Chem. Technol. 2018, 46, 1342–1351. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Ipsakis, D.K.; Karakoulia, S.A.; Kalogiannis, K.G.; Yiannoulakis, H.; Triantafyllidis, K.S.; Lappas, A.A. Isomerization of glucose into fructose over natural and synthetic MgO catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16459–16470. [Google Scholar] [CrossRef]
- Montassier, C.; Giraud, D.; Barbier, J. Polyol conversion by liquid phase heterogeneous catalysis over metals. Stud. Sur. Sci. Catal. 1988, 41, 165–170. [Google Scholar]









| Sample | Ms (emu.g−1) | Mr (emu.g−1) | Mr/Ms | Hc (Oe) |
|---|---|---|---|---|
| CuMgFe-2LDO | 11.70 | 0.91 | 0.08 | 26.71 |
| CuMgFe-3LDO | 11.82 | 1.41 | 0.12 | 27.86 |
| CuMgFe-4LDO | 16.30 | 0.41 | 0.03 | 13.45 |
| CuMgFe-5LDO | 10.00 | 1.38 | 0.14 | 26.20 |
| Catalyst | Cu (mol%) a | Cu/(Mg + Fe) Atomic Ratio a | Base Sites Amounts | ||
|---|---|---|---|---|---|
| W b + M b (µmol/g) | S b (µmol/g) | Total Amount (µmol/g) | |||
| CuMgFe-2LDO | 5.08 | 0.289 | 60.1 | 53.6 | 113.7 |
| CuMgFe-3LDO | 5.01 | 0.290 | 84.2 | 61.4 | 145.6 |
| CuMgFe-4LDO | 5.26 | 0.325 | 109.0 | 90.5 | 199.5 |
| CuMgFe-5LDO | 4.92 | 0.278 | 83.9 | 45.6 | 129.5 |
| Catalyst | Conversion (%) | Selectivity | |
|---|---|---|---|
| 1,2-PDO (%) | Others (%) b | ||
| CuMgFe-2LDO | 38.0 | 96.9 | 3.1 |
| CuMgFe-3LDO | 41.3 | 97.0 | 3.0 |
| CuMgFe-4LDO | 47.8 | 97.5 | 2.5 |
| CuMgFe-5LDO | 37.5 | 96.2 | 3.4 |
| Temperature (°C) | Conversion (%) | Selectivity | |
|---|---|---|---|
| 1, 2-PDO (%) | Others (%) b | ||
| 180 | 47.8 | 97.5 | 2.5 |
| 190 | 60.5 | 97.2 | 2.8 |
| 200 | 75.3 | 96.5 | 3.5 |
| Recycles | Conversion (%) | Selectivity | Composition c | |||
|---|---|---|---|---|---|---|
| 1, 2-PDO (%) | Others b (%) | Cu (mol %) | Mg (mol %) | Fe (mol %) | ||
| 1 | 47.8 | 97.5 | 2.5 | 7.23 | 72.98 | 19.79 |
| 2 | 46.9 | 97.4 | 2.6 | -- | -- | -- |
| 3 | 46.6 | 97.3 | 2.7 | -- | -- | -- |
| 4 | 46.1 | 97.4 | 2.6 | -- | -- | -- |
| 5 | 44.3 | 97.2 | 2.8 | 7.15 | 73.85 | 19.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhang, F.; Wang, Y.; Cheng, D. Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol. Catalysts 2021, 11, 232. https://doi.org/10.3390/catal11020232
Yu X, Zhang F, Wang Y, Cheng D. Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol. Catalysts. 2021; 11(2):232. https://doi.org/10.3390/catal11020232
Chicago/Turabian StyleYu, Xiaopeng, Fubao Zhang, Yi Wang, and Dejun Cheng. 2021. "Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol" Catalysts 11, no. 2: 232. https://doi.org/10.3390/catal11020232
APA StyleYu, X., Zhang, F., Wang, Y., & Cheng, D. (2021). Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol. Catalysts, 11(2), 232. https://doi.org/10.3390/catal11020232