1. Introduction
Gelatin is a peptide mixture resulting from the denaturation of collagen, the main structural protein in connective tissue. Collagen consists of a triple helix of peptides of around 100 kDa (α-chains), assembled in the extracellular matrix into aggregates forming fibrils [
1]. Disruption of the triple helix structure turns very insoluble collagen into soluble gelatin, a more tractable material that has found many applications in the food, pharmaceutical, and biomedical industries [
2,
3,
4].
Sources of commercial gelatin comprise mostly pig and cattle bones, skins, and hides, but growing interest exists in fish gelatin. On the one hand, as a substitute of terrestrial animals for cultural reasons, but also as properties of fish gelatins, different to those of mammalian counterparts, may better suit particular applications [
2,
3]. As a result, many works have dealt with the extraction and properties of gelatin from a wide range of fish species [
5,
6].
By-products of the fishing industry rich in collagen may turn into raw materials for gelatin extraction, especially skins which may contain four-fold more collagen than heads or bones [
7]. This biomass is currently discarded or used to produce low-value fish feed, and may pose environmental problems as its volume expands along with growing consumption of fish, increasingly processed as modern societies demand convenience food, such as fish fillets. In this line, valorization of processing by-products from highly demanded species, such as salmon, is particularly relevant.
A number of previous studies have focused on the isolation of gelatin from salmon skin, looking at the effect of different pretreatments and thermal extraction conditions on yield and properties of the resulting gelatin. The treatments commonly applied involve an alkaline step followed by acid [
8,
9,
10,
11,
12], although some authors have tried initial saline treatment [
7], sometimes followed by alkaline medium and trypsin [
13,
14], or trypsin alone [
14]. Generally, harsher treatments and high extraction temperatures increase yield but result in gelatin with weaker rheological properties, which seem to be influenced by the content of imino acids, hydrophopic amino acids, and molecular weight. Despite the importance of the latter, studies to date have only used relative methods to assess the
Mw of each gelatin fraction, commonly electrophoresis, or capillary viscometry, techniques that only provide a mean value for the whole material.
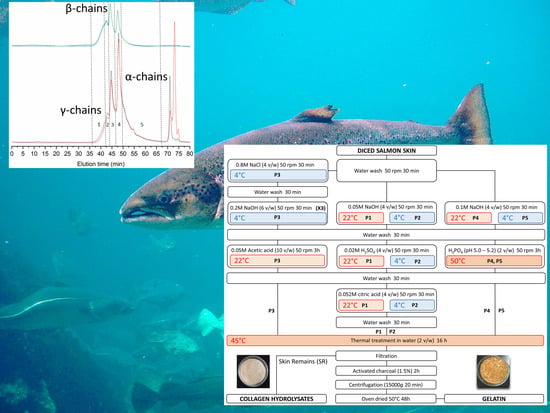
In the present work, we have extracted gelatin from farmed salmon skin using different methods, and studied for the first time the molecular weight distributions of each material by gel permeation chromatography (GPC) with light scattering detection, allowing for absolute molecular weight measurements. The rheological properties of gelatin are discussed in light of this information, along with amino acid profiling. Furthermore, we propose to complete the valorization of salmon skin with the production protein hydrolysates from the solids remaining from gelatin extraction.
4. Discussion
All protocols yield comparable amounts of gelatin (4.51–5.07%,
Table 3), despite the differences in the chemical treatments and associated temperatures. This is unexpected, as previous studies have found significant effects of acid concentration, pretreatment temperature, and extraction temperature on gelatin yield from salmon skin [
9,
13]. The yield values obtained here agree well with a previous work reporting 5.5% yield (estimated based on a 15.3% yield in the skin separated from the muscle remaining in the salmon filleting by-product, with 35.7% skin and 64.3% muscle) [
8]. Other works calculate yield based on hydroxyproline content, making comparison with the present data difficult. Furthermore, yield figures vary widely from 22.4% to 74% [
7,
9,
13,
14].
On the other hand, differences in chemical treatment do affect the gel strength of the resulting gelatins, to the point that P3 does not gel at all, while P1 and P5 reach similar values (98 and 92.5, respectively,
Table 3). These results align with others previously reported [
8,
11,
12]. Of note, treatment temperature shows opposite effects in P1 and P2 compared to P4 and P5. The latter only differ in the temperature of the alkaline treatment, which may indicate that low temperature enhances gel strength. If this holds true for P1 and P2, then the positive effect of risen temperature in the acidic treatments possibly compensates for the deleterious effect on NaOH treatment, as temperature remains constant throughout all three steps (NaOH, H
2SO
4, and citric acid). However, the higher gel strength seen after alkaline treatment at 4 °C, and acidic treatments at 22 °C cannot be generalized in light of the null gel strength of P3 (NaCl and NaOH at 4 °C, and acetic acid at 22 °C).
Processing conditions show scant impact on the aminoacid profile of gelatin (
Table 4). The main aminoacids in collagen (glycine, proline, and hydroproline) varied little across treatments, and while differences in proline content are statistically significant, the values range only from 10.20% to 11.39%. Although P3, which did not gel, contains the lowest amount of proline and protein content, no relationship with gel strength is apparent for the other samples. Hence, aminoacid composition does not explain differences in gel strength.
Molecular weight distributions, however, appear influenced by the process (
Figure 1,
Table 5), and related to differences seen in gel strength. In the strongest gel forming gelatins (P1 and P5), structural integrity is evidenced by distinct peaks of estimated
Mw slightly above 300 kDa, 200 kDa, and 100 kDa, corresponding to the structural units of collagen (
γ-,
β-, and
α-chains, respectively). Higher
Mw species are due to supramolecular aggregates of the former. On the other hand, the only gelatin not capable of gelling (P5) only contains 6% of α-chains, the bulk of the material made of peptides below 100 kDa. These possibly consist of collagen fragments and aminoacids resulting from its degradation, and other non-collagenous proteins. Beyond these extreme cases, we failed to find a clear relationship between gel strength and the proportion of each structural unit. The behavior of intermediate strength gelatins illustrates this fact: while gel strength was slightly superior in P2 (53.5) than in P4 (44.5), around two thirds of P2 consist of protein below 100 kDa, whereas in P4 this fraction accounts for only one third.
Based on the above results, we selected P1 as the preferred process to recover gelatin from salmon skin, and focused on this material for further characterization. As a starting point, we were interested in assessing the thermal stability of the gelatin sample. Weight loss determined by TGA (
Figure 2a) behaved fairly similarly to other gelatins [
42]. Furthermore, the high glass transition temperature determined by DSC (
Figure 2b) suggests that the chain interactions are strong; this transition is related to the
Mw and the chain architecture.
Tg is a non-kinetic event, related to the mobility of the chains; therefore, depending on the solvent used, it may have a different Tg value, as reported in the literature [
42]. Moreover, in DSC an endothermic peak appears around 300 °C, which corroborates the degradation of the low molecular weight protein fraction, as well as structurally bound water, which is observed in the thermogravimetric analysis.
The spectral FTIR profiles of gelatin sample P1 (
Figure 3a) are typical of fish gelatin and comparable to others previously reported for other fish species [
43]. We focused in particular in the Amide I band, as it is more sensitive to changes in the secondary structure. Qualitatively, the Amide I band can be explained by the superposition of bands corresponding to different conformational states of the polypeptide chain [
43]. By applying the second derivative method (
Figure 3b), we identified components (
Table 6) assigned to the triple-helix conformation typical of tropocollagen, in agreement with the GPC elution profile. Furthermore, the presence of a –COOH band confirms that gelatin gelling is responsible for the situation wherein a certain number of carboxylic acid functions are blocked to form
β-sheets and
β-turns, thus forming a supramolecular network. The most intense signal corresponds to random coil disposition, indicating the predominance of a disordered structure. This may indicate that not only the peptides below 100 kDa (38.5%) contribute to this signal, but as do collagen structural units such as α-chains (28%), as determined by GPC (
Table 5).
The rheological measurements of a hydrogel formed with gelatin P1 show typical gel behavior, which disappears as gelatin is heated above 14 °C (
Figure 5). At this point, the network structure formed during gelatinization is disrupted. Previous works have reported similar values (15 °C) [
12], but also lower (10–11 °C) [
8], differences that may be influenced by the diverse extraction procedures used.
The data presented here portrays gelatin as a viable option to add value to skin by-products resulting from fish filleting. However, the extracted gelatin only represents around 5% of the initial biomass (
Table 3), leaving the bulk of the material as waste. To complete the valorization cycle, we propose the proteolysis of these skin remains. In the best case tested, Alcalase induces almost complete conversion of the substrate, leaving only around 5% of undigested material (
Table 7). Concomitantly, the process manages to recover a significant amount of fish oil (up to almost 6%).
The hydrolysates still contain some collagenous material, as shown by the presence of proline and hydroxyproline (almost exclusive to collagen). The contribution of other proteins raises the ratio of essential aminoacids to 41.3%, comparable to hydrolysates prepared from other species [
44,
45,
46], with digestibilities of up to 85.9%. Furthermore, in vitro antihypertensive activity in the Alcalase hydrolysate CH2 (82.3% inhibition; 71.2 µg protein/mL-IC
50) compares favorably with hydrolysates from salmon heads (71.9% inhibition; 478.5 µg protein/mL-IC
50) and frames and trimmings (87.0% inhibition; 653.7 µg protein/mL-IC
50) [
20]. These properties pose the hydrolysates as potential ingredients of food supplements, aquaculture feed, pet food, and microbial culture media.
Digestibility and antihypertensive activity (
Table 7) appear correlated with the
Mw of the hydrolysates, with more fragmented protein possessing higher digestibility and antihypertensive activity. However, previous studies have reported contradictory results, finding direct [
47], inverse [
48], or no relationship [
20,
32].