Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer
Abstract
:1. Introduction
2. Experiments
2.1. Chemistry
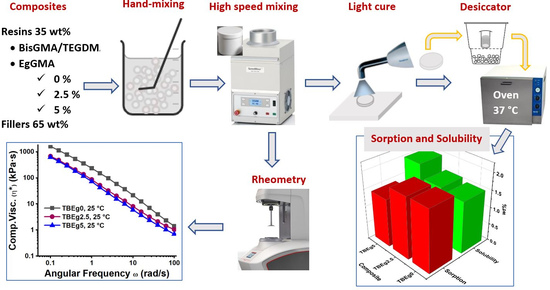
2.2. Preparation of TBEg Experimental Composites
2.3. Water Sorption and Solubility Tests
2.4. Degree of Conversion
2.5. Rheological Measurement
2.6. Statistical Analysis
3. Results and Discussion
3.1. Rheological Analysis of Uncured Composites
3.2. Degree of Conversion, DC
3.3. Water Sorption and Water Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almaroof, A.; Rojo, L.; Mannocci, F.; Deb, S. A resin composite material containing an eugenol derivative for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaroof, A.; Niazi, S.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, L.; Vazquez, B.; Parra, J.; López Bravo, A.; Deb, S.; San Roman, J. From natural products to polymeric derivatives of “eugenol”: A new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Deb, S. Polymer therapeutics in relation to dentistry. Biomater. Oral Craniomaxillofacial Appl. 2015, 17, 13–21. [Google Scholar]
- Almaroof, A.; Niazi, S.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.R.; Siqueira, J.F., Jr.; Ricucci, D.; Lopes, W.S. Dentinal tubule infection as the cause of recurrent disease and late endodontic treatment failure: A case report. J. Endod. 2012, 38, 250–254. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, S.; Dalle Vacche, S.; Vitale, A.; Ladmiral, V.; Caillol, S.; Bongiovanni, R.; Lacroix-Desmazes, P. Photoinduced Polymerization of Eugenol-Derived Methacrylates. Molecules 2020, 25, 3444. [Google Scholar] [CrossRef]
- Rojo, L.; Vázquez, B.; Deb, S.; San Román, J. Eugenol derivatives immobilized in auto-polymerizing formulations as an approach to avoid inhibition interferences and improve biofunctionality in dental and orthopedic cements. Acta Biomater. 2009, 5, 1616–1625. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Saeed, W.S.; Ahmed, A.Y.B.H.; Alrahlah, A.; Al-Kahtani, A.; Aouak, T. New Monomer Based on Eugenol Methacrylate, Synthesis, Polymerization and Copolymerization with Methyl Methacrylate-Characterization and Thermal Properties. Polymers 2020, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Al-Odayni, A.-B.; Alotaibi, D.H.; Saeed, W.S.; Al-Kahtani, A.; Assiri, A.; Alkhtani, F.M.; Alrahlah, A. Eugenyl-2-Hydroxypropyl Methacrylate-Incorporated Experimental Dental Composite: Degree of Polymerization and In Vitro Cytotoxicity Evaluation. Polymers 2022, 14, 277. [Google Scholar] [CrossRef]
- Prakash, P.; Gupta, N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J. Physiol. Pharmacol. 2005, 49, 125. [Google Scholar]
- Saltan, F. Preparation of poly(eugenol-co-methyl methacrylate)/polypropylene blend by creative route approach: Structural and thermal characterization. Iran. Polym. J. 2021, 30, 1227–1236. [Google Scholar] [CrossRef]
- Sokolnicki, T.; Franczyk, A.; Janowski, B.; Walkowiak, J. Synthesis of Bio-Based Silane Coupling Agents by the Modification of Eugenol. Adv. Synth. Catal. 2021, 363, 5493–5500. [Google Scholar] [CrossRef]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and toxicological properties of eugenol. Turk. J. Pharm. Sci. 2017, 14, 201. [Google Scholar] [CrossRef]
- Kaufman, T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Rahim, N.H.C.A.; Asari, A.; Ismail, N.; Osman, H. Synthesis and antibacterial study of eugenol derivatives. Asian J. Chem. 2017, 29, 22. [Google Scholar] [CrossRef]
- Martins, R.M.; Farias, M.D.A.; Nedel, F.; de Pereira, C.M.; Lencina, C.; Lund, R.G. Antimicrobial and cytotoxic evaluation of eugenol derivatives. Med. Chem. Res. 2016, 25, 2360–2367. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Langlois, V.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mater. Today Commun. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lung, C.Y.K.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Saeed, W.S.; Khan, R.; Al-Kahtani, A.; Aouak, T.; Almutairi, K.; Alrahlah, A. Viscosity, Degree of Polymerization, Water Uptake, and Water Solubility Studies on Experimental Dichloro-BisGMA-Based Dental Composites. Appl. Sci. 2021, 11, 3577. [Google Scholar] [CrossRef]
- Davidson, C.; Feilzer, A. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef]
- Alrahlah, A.; Al-Odayni, A.-B.; Al-Mutairi, H.F.; Almousa, B.M.; Alsubaie, F.S.; Khan, R.; Saeed, W.S. A Low-Viscosity BisGMA Derivative for Resin Composites: Synthesis, Characterization, and Evaluation of Its Rheological Properties. Materials 2021, 14, 338. [Google Scholar] [CrossRef]
- Rai, R.U.; Singbal, K.P.; Parekh, V. The effect of temperature on rheological properties of endodontic sealers. J. Conserv. Dent. JCD 2016, 19, 116. [Google Scholar] [CrossRef] [Green Version]
- Metalwala, Z.; Khoshroo, K.; Rasoulianboroujeni, M.; Tahriri, M.; Johnson, A.; Baeten, J.; Fahimipour, F.; Ibrahim, M.; Tayebi, L. Rheological properties of contemporary nanohybrid dental resin composites: The influence of preheating. Polym. Test. 2018, 72, 157–163. [Google Scholar] [CrossRef]
- Tarumi, H.; Imazato, S.; Ehara, A.; Kato, S.; Ebi, N.; Ebisu, S. Post-irradiation polymerization of composites containing bis-GMA and TEGDMA. Dent. Mater. 1999, 15, 238–242. [Google Scholar] [CrossRef]
- Silikas, N.; Eliades, G.; Watts, D. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent. Mater. 2000, 16, 292–296. [Google Scholar] [CrossRef]
- Giannini, M.; Di Francescantonio, M.; Pacheco, R.; Boaro, L.C.; Braga, R. Characterization of water sorption, solubility, and roughness of silorane-and methacrylate-based composite resins. Oper. Dent. 2014, 39, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.K.; Kam Hepdeniz, O.; Esencan Turkaslan, B.; Gurdal, O. The effect of functionalized titanium dioxide nanotube reinforcement on the water sorption and water solubility properties of flowable bulk-fill composite resins. Odontology 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.; Craig, R.G. Correlation of parameters used to estimate monomer conversion in a light-cured composite. J. Dent. Res. 1988, 67, 932–937. [Google Scholar] [CrossRef]
- Sadeghian, H.; Seyedi, S.M.; Saberi, M.R.; Arghiani, Z.; Riazi, M. Design and synthesis of eugenol derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2008, 16, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Bohaty, B.S.; Ye, Q.; Misra, A.; Sene, F.; Spencer, P. Posterior composite restoration update: Focus on factors influencing form and function. Clin. Cosmet. Investig. Dent. 2013, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- AlShaafi, M.M. Factors affecting polymerization of resin-based composites: A literature review. Saudi Dent. J. 2017, 29, 48–58. [Google Scholar] [CrossRef]


| Composite | Resins (wt%) | Fillers (wt%) | Initiation System (wt%, with Respect to the Total Monomers) | ||||
|---|---|---|---|---|---|---|---|
| TEGDMA | BisGMA | EgGMA | S-HA | S-ZrO2 | Initiator, CQ | Co-Initiator, DMAEMA | |
| TBEg0 | 17.50 | 17.50 | 0.00 | 27.86 | 37.14 | 0.5 | 1.0 |
| TBEg2.5 | 16.25 | 16.25 | 2.50 | 27.86 | 37.14 | 0.5 | 1.0 |
| TBEg5 | 15.00 | 15.00 | 5.00 | 27.86 | 37.14 | 0.5 | 1.0 |
| Composite | DC %; n = 3 | Complex Viscosity η*, (kPa·s) at ω = 1.0 (rad/s); n = 3 | WSP (wt%); n = 5 | WSL (wt%); n = 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 37 °C | 1st Cycle | 2nd Cycle | 3rd Cycle | 1st Cycle | 2nd Cycle | 3rd Cycle | ||
| TBEg0 | 68.75 a (0.750) | 234.70 A,a (13.40) | 52.01 B,a (9.64) | 1.837 A,a (0.172) | 2.975 B,a (0.113) | 3.072 BC,a (0.050) | 1.505 A,a (0.174) | 0.072 B,a (0.051) | 0.018 BC,a (0.005) |
| TBEg2.5 | 67.17 a (1.250) | 86.44 A,b (16.47) | 51.81 B,a (8.71) | 1.819 A,a (0.153) | 3.081 B,a (0.172) | 2.933 BC,a (0.134) | 1.590 A,a (0.227) | 0.017 B,b (0.011) | 0.042 BC,a (0.043) |
| TBEg5 | 58.01 b (1.018) | 72.34 A,bc (17.14) | 30.59 B,b (0.09) | 1.409 A,b (0.189) | 3.013 B,a (0.107) | 2.728 C,a (0.121) | 1.982 A,b (0.079) | 0.009 Bb,c (0.009) | 0.006 BC,a (0.027) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrahlah, A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alkhtani, F.M.; Al-Maflehi, N.S. Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer. Polymers 2022, 14, 366. https://doi.org/10.3390/polym14030366
Alrahlah A, Al-Odayni A-B, Saeed WS, Al-Kahtani A, Alkhtani FM, Al-Maflehi NS. Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer. Polymers. 2022; 14(3):366. https://doi.org/10.3390/polym14030366
Chicago/Turabian StyleAlrahlah, Ali, Abdel-Basit Al-Odayni, Waseem Sharaf Saeed, Abdullah Al-Kahtani, Fahad M. Alkhtani, and Nassr S. Al-Maflehi. 2022. "Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer" Polymers 14, no. 3: 366. https://doi.org/10.3390/polym14030366
APA StyleAlrahlah, A., Al-Odayni, A. -B., Saeed, W. S., Al-Kahtani, A., Alkhtani, F. M., & Al-Maflehi, N. S. (2022). Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer. Polymers, 14(3), 366. https://doi.org/10.3390/polym14030366