Milk Composition of Free-Ranging Impala (Aepyceros melampus) and Tsessebe (Damaliscus lunatus lunatus), and Comparison with Other African Bovidae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Determination Water Content
2.3. Protein Analysis
2.4. Lipid Analysis
2.5. Carbohydrate Analysis
2.6. Determination of Energy
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Proteins
4.2. Carbohydrates
4.3. Lipids
4.4. Energy
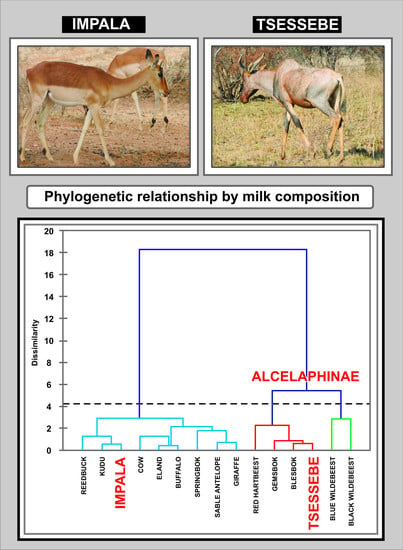
4.5. Statistical Analysis and Interspecies Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jennes, R.; Patton, S. Principles of Dairy Chemistry; Wiley & Son: New York, NY, USA, 1976. [Google Scholar]
- Oftedal, O.T. Milk composition, milk yield and energy output at peak lactation: A comparative review. Symp. Zool. Soc. Lond. 1984, 51, 33–85. [Google Scholar]
- Casoli, C.; Duranti, E.; Morbidini, L.; Panella, F.; Vizioli, V. Quantitative and compositional variations of Massesse sheep milk by parity and stage of lactation. Small Rumin. Res. 1989, 2, 47–62. [Google Scholar] [CrossRef]
- Voutsinas, L.; Pappas, C.; Katsiari, M. The composition of Alpine goats’ milk during lactation in Greece. J. Dairy Res. 1990, 57, 41–51. [Google Scholar] [CrossRef]
- Holand, Ø.; Gjøstein, H.; Nieminen, M. Reindeer Milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: London, UK, 2006; pp. 355–370. [Google Scholar]
- Osthoff, G.; Hugo, A.; De Wit, M.; Nguyen, T.P.M. The chemical composition of milk from free-ranging African buffalo (Syncerus caffer). S. Afr. J. Wildl. Res. 2009, 39, 97–102. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Wit, M. Comparison of the milk composition of free-ranging blesbok, black wildebeest and blue wildebeest of the subfamily Alcelaphinae (family: Bovidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 154, 48–54. [Google Scholar] [CrossRef]
- Petzinger, C.; Oftedal, O.T.; Jacobsen, K.; Murtough, K.L.; Irlbeck, N.A.; Power, M.L. Proximate composition of milk of the bongo (Tragelaphus eurycerus) in comparison to other African bovids and to hand-rearing formulas. Zoo Biol. 2014, 33, 305–313. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Wit, M. Comparison of the milk composition of free-ranging eland and kudu and gemsbok and scimitar oryx with observations on lechwe, okapi and Southern pudu. Afr. J. Wildlife Res. 2012, 42, 23–34. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Wit, M. Milk composition of free-ranging springbok (Antidorcas marsupialis). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 421–426. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; De Wit, M. Milk composition of free-ranging sable antelope (Hippotragus niger). Mamm. Biol. 2007, 72, 116–122. [Google Scholar] [CrossRef]
- Osthoff, G.; Hugo, A.; Madende, M.; Deacon, F.; Nel, P.J. Milk composition of free-ranging red hartebeest, giraffe, Southern reedbuck and warthog and a phylogenetic comparison of the milk of African Artiodactyla. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 204, 93–103. [Google Scholar] [CrossRef]
- Neville, M.C. Physiology of lactation. Clin. Perinatol. 1998, 26, 251–279. [Google Scholar] [CrossRef]
- Hood, R.H.; Kunz, T.H.; Oftedal, O.T.; Iverson, S.J.; Le Blanc, D.; Seyagat, J. Interspecific and intraspecific variation in proximate, mineral and fatty acid composition of milk in old world fruit bats (Chiroptera: Pteropodidae). Physiol. Biochem. Zool. 2001, 74, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Milligan, L.A.; Rappoport, S.I.; Cranfield, M.R.; Dittus, W.; Glander, K.E.; Oftedal, O.T.; Power, M.L.; Whittier, C.A.; Bazinet, R.P. Fatty acid composition of wild anthropoid primate milks. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 149, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Haenlein, G.F.W.; Wendorff, W.L. Sheep Milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: London, UK, 2006; pp. 137–194. [Google Scholar]
- Park, Y.W. 2006. Goat Milk—Chemistry and Nutrition. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: London, UK, 2006; pp. 34–58. [Google Scholar]
- Talpur, F.N.; Bhanger, M.I.; Memon, N.N. Milk fatty acid composition of indigenous goat and ewe breeds from Sindh, Pakistan. J. Food Compos. Anal. 2009, 22, 59–64. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Zervas, G. Comparative study between sheep and goats on rumenic acid and vaccenic acid in milk fat under the same dietary treatments. Livest. Sci. 2008, 119, 87–94. [Google Scholar] [CrossRef]
- Myburgh, J.; Osthoff, G.; Hugo, A.; De Wit, M.; Nel, K.; Fourie, D. Comparison of the milk composition of free ranging indigenous African cattle breeds. S. Afr. J. Animal Sci. 2012, 42, 1–14. [Google Scholar] [CrossRef]
- Lkhagvajav, B. Lipido-acidic composition of milk fat of Mongolian yak and khaihags. J. Food. Technol. 1978, 4, 41–43. [Google Scholar]
- Jensen, R.G. Handbook of Milk Composition; Academic Press: London, UK, 1995. [Google Scholar]
- Pandya, A.J.; Khan, M.M. Buffalo Milk Utilization for Dairy Products. Reindeer milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Eds.; Blackwell: London, UK, 2006; pp. 215–256. [Google Scholar]
- Iverson, S.J.; Oftedal, O.T. Phylogenetic and Ecological Variation in the Fatty Acid Composition of Milks. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: New York, NY, USA, 1995; pp. 790–827. [Google Scholar]
- Dill, C.W.; Tybor, P.T.; McGill, R.; Ramsey, C.W. Gross composition and fatty acid constitution of blackbuck antelope (Antilope cervicapra) milk. Can. J. Zool. 1972, 50, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.L.; Jenness, R. Comparative biochemical studies of milk—VI. Constituent fatty acids of milk fats of addisional species. Comp. Biochem. Physiol. 1971, 38, 353–359. [Google Scholar]
- Martin, P.; Cebo, C.; Miranda, G. Interspecies Comparison of Milk Proteins: Quantitative Variability and Molecular Diversity. In Advanced Dairy Chemistry, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2013; pp. 387–429. [Google Scholar]
- Bleck, G.T.; Bremel, R.D. Correlation of the α-lactalbumin (+15) polymorphism to milk production of Holsteins. J. Dair Sci. 1993, 76, 2292–2298. [Google Scholar] [CrossRef]
- Moro, G.; Minoli, I.; Mosca, M.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 291–295. [Google Scholar] [CrossRef]
- Vestweber, D.; Blanks, J. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999, 79, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, M.J.; Rudloff, S.; Kung, C.; Kime, R.K.H. Investigations of the in vitro transport of human milk oligosaccharides by a Caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J. Biol. Chem. 2001, 276, 34363–34370. [Google Scholar] [CrossRef] [Green Version]
- Oftedal, O.T.; Iverson, S.J. Comparative Analysis of Non-Human milks: Phylogenetic Variation in the Gross Composition of Milks. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: New York, NY, USA, 1995; pp. 749–788. [Google Scholar]
- Skibiel, A.M.; Downing, L.M.; Orr, T.J.; Hood, W.R. The evolution of the nutrient composition of mammalian milks. J. Animal Ecol. 2013, 82, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Skinner, J.D.; Smithers, R.H.N. The Mammals of the Southern African Subregion, 2nd ed.; University of Pretoria: Pretoria, South Africa, 1990. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Leco Corporation. FP-528 Protein/Nitrogen Determinator. FP-528 Instruction Manual, Version 1.2.; Leco® Corporation: Geleen, The Netherlands, 2001. [Google Scholar]
- Igarashi, Y. An improved procedure for the preliminary fractionation of milk proteins. Int. Dairy J. 1995, 5, 305–310. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Park, P.W.; Goins, R.E. In Situ Preparation of Fatty Acid Methyl Esters for Analysis of Fatty Acid Composition in Foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Perrin, D.R. The calorific value of milk of different species. J.Dairy Res. 1958, 25, 215–220. [Google Scholar] [CrossRef]
- NCSS Statistical Systems. Statistical System for Windows; NCSS: Kaysville, UT, USA, 2007. [Google Scholar]
- Smit, L.E.; Schönfeldt, H.C.; De Beer, W.H.J.; Smith, M.F. The effect of locality and season on the composition of South African whole milk. J. Food. Comp. Anal. 2000, 13, 345–368. [Google Scholar] [CrossRef]
- Pirisi, A.; Piredda, G.; Papoff, C.M.; Salvo, R.; Di Pintus, S.; Garro, G.; Ferranti, P.; Chianese, L. Effects of sheep alphas1 casein CC, CD and DD genotypes on milk composition and cheese making properties. J. Dairy. Res. 1999, 66, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Simos, E.; Voutsinas, L.P.; Pappas, C.P. Composition of milk of native Greek goats in the region of Metsovo. Small Rumin. Res. 1991, 4, 47–60. [Google Scholar] [CrossRef]
- Ben Shaul, D.M. The composition of the milk of wild animals. Int. Zoo Yearb. 1962, 4, 333–343. [Google Scholar]
- Oftedal, O.T.; Eisert, R.; Barrell, G.K. Comparison of analytical and predictive methods for water, protein, fat, sugar and gross energy in marine mammal milk. J. Dairy Sci. 2014, 97, 4713–4732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, M.H.; Vrba, E.S. A complete estimate of the phylogenetic relationships in Ruminantia: A dated species-level supertree of the extant ruminants. Biol. Rev. 2005, 80, 269–302. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Sánchez, N.R.; Spörndly, E.; Ledin, I. Effect of feeding different levels of foliage of Moringa oleifera to creole dairy cows on intake, digestibility, milk production and composition. Livest. Sci. 2006, 101, 24–31. [Google Scholar] [CrossRef]
- Woods, V.B.; Fearon, A.M. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Acocks, J.P.H. Veld Types of South Africa; Memoirs of the Botanical Survey of South Africa; Botanical Search Institute: Pretoria, South Africa, 1988; Volume 57. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland, 19th ed.; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- De Peters, E.J.; German, J.B.; Taylor, S.J.; Essex, S.T.; Perez-Monti, H. Fatty acid and triglyceride composition of milk fat from lactating Holstein cows in response to supplemental canola oil. J. Dairy Sci. 2001, 84, 929–936. [Google Scholar] [CrossRef]
- Loor, J.J.; Ferlay, A.; Ollier, A.; Doreau, M.; Chillard, Y. Relationship among trans and conjugated fatty acids and bovine milk fat yield due to dietary concentrate and linseed oil. J. Dairy Sci. 2005, 88, 726–740. [Google Scholar] [CrossRef] [Green Version]
- Sikes, R.S.; The Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mamm. 2016, 97, 663–688. [Google Scholar] [CrossRef]


| Nutrient (g/100 g Milk) | Impala | Tsessebe | Sheep [43] | Goat [44] | Cow [1] | Impala [45] |
|---|---|---|---|---|---|---|
| n/N | 20/10 | 3/3 | ||||
| Moisture | 85.37 ± 2.28 | 82.73 ± 1.76 | 83.2 | 87.2 | 87.1 | - |
| Fat | 5.56 ± 1.96 | 8.44 ± 3.19 | 7.0 | 5.2 | 3.9 | 20.4 |
| FFDM | 9.07 ± 1.41 | 8.82 ± 1.49 | - | - | - | - |
| Protein | 6.60 ± 0.51 | 5.15 ± 0.49 | 5.3 | 3.56 | 3.27 | 10.8 |
| Whey | 1.43 ± 0.60 | 0.56 ± 0.17 | 1.04 | 0.76 | 0.63 | - |
| Casein | 5.09 ± 0.64 | 4.59 ±0.34 | 4.26 | 2.80 | 2.6 | - |
| NPN | 0.09 ± 0.04 | 0.08 ± 0.02 | - | - | - | - |
| Lactose | 4.36 ± 0.94 | 6.10 ± 3.85 | 4.30 | 4.20 | 4.8 | 2.4 |
| Galctose | ND | 0.08 ± 0.07 | - | - | 0.05 | - |
| Oligosaccharide | ND | ND | - | - | - | - |
| Gross energy (kcal/g) | 36.63 ± 4.51 | 45.17 ± 5.44 | - | - | - | - |
| Species | Impala | Tsessebe | ||
|---|---|---|---|---|
| n/N | 20/10 | 3/3 | ||
| FAME (% of Total Fatty Acids) | ||||
| Butyric | C4:0 | 1.23 ± 0.37 | 0.79 ± 0.04 | |
| Caproic | C6:0 | 0.46 ± 0.19 | 1.89 ± 0.14 | |
| Caprylic | C8:0 | 0.19 ± 0.31 | 9.53 ± 0.22 | |
| Capric | C10:0 | 0.96 ± 0.34 | 11.49 ± 1.15 | |
| Hendecanoic | C11:0 | ND | ND | |
| Lauric | C12:0 | 3.55 ± 0.85 | 3.98 ± 0.23 | |
| Tridecoic | C13:0 | 0.06 ± 0.04 | 0.06 ± 0.01 | |
| Myristic | C14:0 | 13.95 ± 2.35 | 14.79 ± 0.9 | |
| Myristoleic | C14:1c9 | 0.15 ± 0.10 | 0.24 ± 0.03 | |
| Pentadecylic | C15:0 | 2.61 ± 1.67 | 1.15 ± 0.1 | |
| Pentadecenoic | C15:1c10 | 0.02 ± 0.02 | 0 ± 0 | |
| Palmitic | C16:0 | 27.89 ± 1.09 | 29.45 ± 2.87 | |
| Palmitoleic | C16:1c9 | 0.48 ± 0.23 | 0.55 ± 0.07 | |
| Margaric | C17:0 | 1.09 ± 0.41 | 0.76 ± 0.14 | |
| Heptadecenoic | C17:1c10 | 0.11 ± 0.11 | 0.08 ± 0.02 | |
| Stearic acid | C18:0 | 18.65 ± 3.15 | 11.78 ± 1.47 | |
| Elaidic | C18:1t9 | 0.31 ± 0.43 | 0.16 ± 0.04 | |
| Oleic | C18:1c9 | 21.45 ± 2.13 | 15.69 ± 3.3 | |
| Vaccenic | C18:1c7 | 2.08 ± 1.00 | 0 ± 0 | |
| Linolelaidic | C18:2t9,12 (n-6) | 0.55 ± 0.34 | 0 ± 0 | |
| Linoleic | C18:2c9,12 (n-6) | 1.76 ± 0.55 | 1.54 ± 0.72 | |
| Conjugated linoleic acid | C18:2c9t11(n-6) | 0.25 ± 0.07 | 0.35 ± 0.15 | |
| α-Linolenic | C18:3c9,12,15 (n-3) | 0.63 ± 0.35 | 0.35 ± 0.15 | |
| γ-Linolenic | C18:3c6,9,12 (n-6) | 0.55 ± 0.36 | ND | |
| Nonadecanoic | C19:0 | 0.16 ± 0.08 | ND | |
| Arachidic | C20:0 | 0.34 ± 0.17 | 0.46 ± 0.05 | |
| Eicosenoic | C20:1c11 | 0.01 ± 0.02 | 0.04 ± 0.01 | |
| Eicosadienoic | C20:2c11,14 (n-6) | 0.01 ± 0.01 | ND | |
| Eicosatrienoic | C20:3c11,14,17 (n-3) | 0.11 ± 0.12 | ND | |
| Eicosatrienoic | C20:3c8,11,14 (n-6) | 0.04 ± 0.05 | 0.24 ± 0.03 | |
| Arachidonic | C20:4c5,8,11,14 (n-6) | 0.13 ± 0.09 | 0.03 ± 0.02 | |
| Eicosopentaenoic | C20:5c5,8,11,14,17 (n-3) | 0.03 ± 0.04 | 0 ± 0 | |
| Heneicosanoic | C21:0 | 0.05 ± 0.04 | 0.15 ± 0.02 | |
| Behenic | C22:0 | ND | ND | |
| Erucic | C22:1c13 | 0.03 ± 0.04 | ND | |
| Docosadienoic | C22:2c13,16 (n-6) | ND | 0.05 ± 0.03 | |
| Docosapentaenoic | C22:5c7,10,13,16,19 (n-3) | 0.03 ± 0.04 | ND | |
| Docosahexanoic | C22:6c4,7,10,13,16,19 (n-3) | ND | ND | |
| Tricosanoic | C23:0 | 0.05 ± 0.05 | 0.16 ± 0.01 | |
| Lignoceric | C24:0 | 0.06 ± 0.05 | 0.13 ± 0.01 | |
| Nervonic | C24:1c15 | 0.01 ± 0.01 | 0.06 ± 0.02 | |
| Fatty acid ratios: | ||||
| Total saturated fatty acids (SFAs) | 71.31 ± 2.58 | 80.24 ± 3.89 | ||
| Total monounsaturated fatty acids (MUFAs) | 24.62 ± 2.17 | 16.82 ± 3.37 | ||
| Total polyunsaturated fatty acids (PUFAs) | 4.08 ± 0.71 | 2.95 ± 0.64 | ||
| Total omega-3 fatty acids | 1.24 ± 0.20 | 2.21 ± 0.72 | ||
| Total omega-6 fatty acids | 2.83 ± 0.81 | 0.74 ± 0.18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osthoff, G.; Hugo, A.; Madende, M.; Schmidt, L.; Kobeni, S.; Deacon, F. Milk Composition of Free-Ranging Impala (Aepyceros melampus) and Tsessebe (Damaliscus lunatus lunatus), and Comparison with Other African Bovidae. Animals 2021, 11, 516. https://doi.org/10.3390/ani11020516
Osthoff G, Hugo A, Madende M, Schmidt L, Kobeni S, Deacon F. Milk Composition of Free-Ranging Impala (Aepyceros melampus) and Tsessebe (Damaliscus lunatus lunatus), and Comparison with Other African Bovidae. Animals. 2021; 11(2):516. https://doi.org/10.3390/ani11020516
Chicago/Turabian StyleOsthoff, Gernot, Arnold Hugo, Moses Madende, Lauren Schmidt, Sibusiso Kobeni, and Francois Deacon. 2021. "Milk Composition of Free-Ranging Impala (Aepyceros melampus) and Tsessebe (Damaliscus lunatus lunatus), and Comparison with Other African Bovidae" Animals 11, no. 2: 516. https://doi.org/10.3390/ani11020516
APA StyleOsthoff, G., Hugo, A., Madende, M., Schmidt, L., Kobeni, S., & Deacon, F. (2021). Milk Composition of Free-Ranging Impala (Aepyceros melampus) and Tsessebe (Damaliscus lunatus lunatus), and Comparison with Other African Bovidae. Animals, 11(2), 516. https://doi.org/10.3390/ani11020516