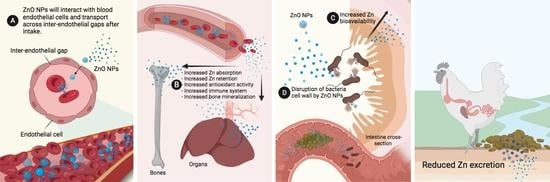
Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation and Characterization of Biosynthesized Zinc Oxide Nanoparticles (ZnO NPs)
2.3. In Vitro Study of ZnO NPs Dissolution in the Simulated Physiological Condition of Poultry GIT System
2.4. Experimental Birds, Husbandry, and Diets
2.5. Slaughtering and Sample Collection
2.6. Acid Digestion and Mineral Determination
2.7. Evaluation of Antioxidative Status
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesized ZnO NPs
3.2. Dissolution of ZnO and Biosynthesized ZnO NPs under the Simulated Physiological Condition of the Chicken GIT System In Vitro
3.3. Zn Uptake and Concentration in Serum, Tibia Bone, Liver, and Breast Tissue
3.4. Fecal Zn Excretion
3.5. Tibia Bone Traits, Ca, and P Retention
3.6. Antioxidative Status
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Y.M.; Choct, M.; Iji, P.A.; Bruerton, K. Trace mineral interactions in broiler chicken diets. Br. Poult. Sci. 2010, 51, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.J.; Zubillaga, M.; Lysionek, A.; Sarabia, M.I.; Caro, R.; De Paoli, T.; Hager, A.; Weill, R.; Boccio, J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000, 20, 737–755. [Google Scholar] [CrossRef]
- Salim, H.M.; Jo, C.; Lee, B.D. Zinc in broiler feeding and nutrition. Avian Biol. Res. 2008, 1, 5–18. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Mbajiorgu, C.A. Potentials of dietary zinc supplementation in improving growth performance, health status, and meat quality of broiler chickens. Biol. Trace Elem. Res. 2022, 200, 1–14. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; Springer: Washington, DC, USA, 1994. [Google Scholar]
- Miles, R.D.; O’Keefe, S.F.; Henry, P.R.; Ammerman, C.B.; Luo, X.G. The effect of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and dietary prooxidant activity. Poult. Sci. 1998, 77, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Vantress, C. Broiler Performance and Nutrition Supplement; Cobb-Vantress Inc.: Siloam Springs, AR, USA, 2008. [Google Scholar]
- Aviagen Ross 308. Broiler: Nutrition Specifications 2019. Aviagen 2019, 1, 1–10. [Google Scholar]
- Nguyen, H.T.T.; Morgan, N.; Roberts, J.R.; Wu, S.B.; Swick, R.A.; Toghyani, M. Zinc hydroxychloride supplementation improves tibia bone development and intestinal health of broiler chickens. Poult. Sci. 2021, 100, 1–9. [Google Scholar] [CrossRef]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock, 3rd ed.; CABI Publishing: Wallingford, UK, 1999. [Google Scholar]
- Mahmoud, U.T.; Abdel-Mohsein, H.S.; Mahmoud, M.A.M.; Amen, O.A.; Hassan, R.I.M.; Abd-El-Malek, A.M.; Rageb, S.M.M.; Waly, H.S.A.; Othman, A.A.; Osman, M.A. Effect of zinc oxide nanoparticles on broilers’ performance and health status. Trop. Anim. Health Prod. 2020, 52, 2043–2054. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef]
- Fatholahi, A.; Khalaji, S.; Hosseini, F.; Abbasi, M. Nano-Bio zinc synthesized by Bacillus subtilis modulates broiler performance, intestinal morphology and expression of tight junction’s proteins. Livest. Sci. 2021, 251, 104660. [Google Scholar] [CrossRef]
- Kumar, A.; Hosseindoust, A.; Kim, M.; Kim, K.; Choi, Y.; Lee, S.; Lee, S.; Lee, J.; Cho, H.; Kang, W.S.; et al. Nano-sized zinc in broiler chickens: Effects on growth performance, zinc concentration in organs, and intestinal morphology. J. Poult. Sci. 2021, 58, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Li, Z.; Li, J.; Chen, Y.; Wang, T.; Wang, C. Effects of zinc oxide nanoparticles on growth, intestinal barrier, oxidative status and mineral deposition in 21-day-old broiler chicks. Biol. Trace Elem. Res. 2022, 200, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Langer, R. Oral particulate delivery: Status and future trends. Adv. Drug Deliv. Rev. 1998, 34, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Hillery, A.M.; Hussain, N.; Jani, P.U. Nanoparticles as carriers for oral peptide absorption: Studies on particle uptake and fate. J. Control. Release 1995, 36, 39–46. [Google Scholar] [CrossRef]
- Kociova, S.; Dolezelikova, K.; Horky, P.; Skalickova, S.; Baholet, D.; Bozdechova, L.; Vaclavkova, E.; Belkova, J.; Nevrkla, P.; Skladanka, J. Zinc phosphate-based nanoparticles as alternatives to zinc oxide in diet of weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- El-Bahr, S.M.; Shousha, S.; Albokhadaim, I.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; El-Garhy, O.; Abdelgawad, A.; El-Naggar, M.; Moustafa, M.; et al. Impact of dietary zinc oxide nanoparticles on selected serum biomarkers, lipid peroxidation and tissue gene expression of antioxidant enzymes and cytokines in Japanese quail. BMC Vet. Res. 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Abedini, M.; Shariatmadari, F.; Karimi Torshizi, M.A.; Ahmadi, H. Effects of zinc oxide nanoparticles on the egg quality, immune response, zinc retention, and blood parameters of laying hens in the late phase of production. J. Anim. Physiol. Anim. Nutr. 2018, 102, 736–745. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, Z.; Yuan, J.; Yang, Y.; Guo, Y.; Zhang, B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J. Microbiol. 2014, 52, 1002–1011. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alaidaroos, B.A.; Farsi, R.M.; Abou-Kassem, D.E.; El-Saadony, M.T.; Saad, A.M.; Shafi, M.E.; Albaqami, N.M.; Taha, A.E.; Ashour, E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals 2021, 11, 1878. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Attia, A.I.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Madkour, M.; Alagawany, M. Use of biological nano zinc as a feed additive in quail nutrition: Biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021, 20, 324–335. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, K.; Zhou, L.; He, J.; Zheng, X.; Zhang, L.; Zhong, X.; Wang, T. Evaluation of long-term toxicity of oral zinc oxide nanoparticles and zinc sulfate in mice. Biol. Trace Elem. Res. 2017, 178, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Milani, N.C.; Sbardella, M.; Ikeda, N.Y.; Arno, A.; Mascarenhas, B.C.; Miyada, V.S. Dietary zinc oxide nanoparticles as growth promoter for weanling pigs. Anim. Feed Sci. Technol. 2017, 227, 13–23. [Google Scholar] [CrossRef]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals 2021, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Gad El-Rab, S.M.F.; Abo-Amer, A.E.; Asiri, A.M. Biogenic synthesis of zno nanoparticles and its potential use as antimicrobial agent against multidrug-resistant pathogens. Curr. Microbiol. 2020, 77, 1767–1779. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.; Karimi, N.; Salimikia, I. Phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J. Ind. Eng. Chem. 2019, 72, 457–473. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Fact. 2020, 19, 10. [Google Scholar] [CrossRef]
- Matin, H.R.H.; Shariatmadari, F.; Torshizi, M.A.K.; Chiba, L.I. Effect of dietary fibre sources on in vitro mineral binding capacity and growth performance, mineral digestibility, tibia and intestinal characteristics in broiler chickens. Europ. Poult. Sci. 2018, 82, 1612–9199. [Google Scholar]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cobb-Vantress. Broiler Guide Management. Available online: https://www.cobb-vantress.com/assets/5c7576a214/Broiler-guide-R1.pdf (accessed on 10 September 2022).
- Qudsieh, R.I.; Smith, D.P.; Brake, J. Effect of elevated dietary inorganic zinc on live performance, carcass yield, and quality of male and female broilers. Poult. Sci. 2018, 97, 4122–4130. [Google Scholar] [CrossRef] [PubMed]
- Güz, B.C.; Molenaar, R.; de Jong, I.C.; Kemp, B.; van Krimpen, M.; van den Brand, H. Effects of green light emitting diode light during incubation and dietary organic macro and trace minerals during rearing on tibia characteristics of broiler chickens at slaughter age. Poult. Sci. 2021, 100, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Laur, N.; Kinscherf, R.; Pomytkin, K.; Kaiser, L.; Knes, O.; Deigner, H.P. ICP-MS trace element analysis in serum and whole blood. PLoS ONE 2020, 15, e0233357. [Google Scholar] [CrossRef] [PubMed]
- Paek, H.J.; Lee, Y.J.; Chung, H.E.; Yoo, N.H.; Lee, J.A.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Yu, J.; Choi, S.J. Fate determination of zno in commercial foods and human intestinal cells. Int. J. Mol. Sci. 2020, 21, 433. [Google Scholar] [CrossRef] [Green Version]
- Youn, S.-M.; Choi, S.-J. Food additive zinc oxide nanoparticles: Dissolution, interaction, fate, cytotoxicity, and oral toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef]
- Baek, M.; Chung, H.E.; Yu, J.; Lee, J.A.; Kim, T.H.; Oh, J.M.; Lee, W.J.; Paek, S.M.; Lee, J.K.; Jeong, J. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int. J. Nanomed. 2012, 7, 3081. [Google Scholar]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Mittag, A.; Hoera, C.; Kämpfe, A.; Westermann, M.; Kuckelkorn, J.; Schneider, T.; Glei, M. Cellular uptake and toxicological effects of differently sized zinc oxide nanoparticles in intestinal cells. Toxics 2021, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, P.; Kaszewski, J.; Dominiak, B.; Damentko, M.; Serafińska, I.; Rosowska, J.; Gralak, M.A.; Krajewski, M.; Witkowski, B.S.; Gajewski, Z. Preliminary studies on biodegradable zinc oxide nanoparticles doped with fe as a potential form of iron delivery to the living organism. Nanoscale Res. Lett. 2019, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Radeloff, K.; Radeloff, A.; Tirado, M.R.; Scherzad, A.; Hagen, R.; Kleinsasser, N.H.; Hackenberg, S. Long-term impact of zinc oxide nanoparticles on differentiation and cytokine secretion of human adipose-derived stromal cells. Materials. 2019, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Fakra, S.C.; Xia, T.; Pokhrel, S.; Mädler, L.; Nel, A.E. The fate of ZnO nanoparticles administered to human bronchial epithelial cells. ACS Nano 2012, 6, 4921–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosharraf, M.; Nyström, C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int. J. Pharm. 1995, 122, 35–47. [Google Scholar] [CrossRef]
- Li, M.Z.; Huang, J.T.; Tsai, Y.H.; Mao, S.Y.; Fu, C.M.; Lien, T.F. Nanosize of zinc oxide and the effects on zinc digestibility, growth performances, immune response and serum parameters of weanling piglets. Anim. Sci. J. 2016, 87, 1379–1385. [Google Scholar] [CrossRef]
- Wang, C.; Xie, P.; Liu, L.L.; Lu, J.J.; Zou, X.T. Effects of dietary capsulated zinc oxide on growth performance, blood metabolism and mineral concentrations in weaning piglets. Asian J. Anim. Vet. Adv. 2013, 8, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Mavromichalis, I.; Emmert, J.L.; Aoyagi, S.; Baker, D.H. Chemical composition of whole body, tissues, and organs of young chickens (Gallus domesticus). J. Food Compos. Anal. 2000, 13, 799–807. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a therapeutic agent in bone regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Mohammadi, V.; Ghazanfari, S.; Mohammadi-Sangcheshmeh, A.; Nazaran, M.H. Comparative effects of zinc-nano complexes, zinc-sulphate and zinc-methionine on performance in broiler chickens. Br. Poult. Sci. 2015, 56, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Burrell, A.L.; Dozier, W.A.; Davis, A.J.; Compton, M.M.; Freeman, M.E.; Vendrell, P.F.; Ward, T.L. Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Br. Poult. Sci. 2004, 45, 225–263. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, S.; Timmons, J.; Ao, T.; Paul, M.; MacAlintal, L.; Pescatore, A.; Cantor, A.; Dawson, K.A. Effect of manganese preconditioning and replacing inorganic manganese with organic manganese on performance of male broiler chicks. Poult. Sci. 2019, 98, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Ali, H.; El-Mandrawy, S. Effects of different zinc sources on performance, bio distribution of minerals and expression of genes related to metabolism of broiler chickens. Zagazig Vet. J. 2017, 45, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Upadhaya, S.D.; Kim, I.H. Importance of micronutrients in bone health of monogastric animals and techniques to improve the bioavailability of micronutrient supplements—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1885–1895. [Google Scholar] [CrossRef] [Green Version]
- Edwards, H.M. Nutrition and skeletal problems in poultry. Poult. Sci. 2000, 79, 1018–1023. [Google Scholar] [CrossRef]
- Bromfield, J.I.; Hoffman, L.C.; Horyanto, D.; Soumeh, E.A. Enhancing growth performance, organ development, meat quality, and bone mineralisation of broiler chickens through multi-enzyme super-dosing in reduced energy diets. Animals 2021, 11, 2791. [Google Scholar] [CrossRef]
- Li, L.; Abouelezz, K.F.M.; Gou, Z.; Lin, X.; Wang, Y.; Fan, Q.; Cheng, Z.; Ding, F.; Jiang, S.; Jiang, Z. Optimization of dietary zinc requirement for broiler breeder hens of Chinese yellow-feathered chicken. Animals 2019, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Shelton, J.L.; Southern, L.L. Interactive effect of zinc, copper and manganese in diets for broilers. Int. J. Poult. Sci. 2007, 6, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Starcher, B.; Kratzer, F.H. Effect of zinc on bone alkaline phosphatase in turkey poults. J. Nutr. 1963, 79, 18–22. [Google Scholar] [CrossRef]
- Stewart, A.K.; Magee, A.C. Effect of zinc toxicity on calcium, phosphorus and magnesium metabolism of young rats. J. Nutr. 1964, 82, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdi, M.; López-Vergé, S.; Manzanilla, E.G.; Barroeta, A.C.; Pérez, J.F. Effect of different levels of calcium and phosphorus and their interaction on the performance of young broilers. Poult. Sci. 2015, 94, 2144–2151. [Google Scholar] [PubMed]
- Onyango, E.M.; Hester, P.Y.; Stroshine, R.; Adeola, O. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 2003, 82, 1787–1791. [Google Scholar] [PubMed]
- Lowe, N.M.; Fraser, W.D.; Jackson, M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002, 61, 181–185. [Google Scholar] [CrossRef]
- Shaker, J.L.; Deftos, L. Calcium and Phosphate Homeostasis. Endocr. Reprod. Physiol. 2018, 1, 77-e1. [Google Scholar]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Hafez, A.; Nassef, E.; Fahmy, M.; Elsabagh, M.; Bakr, A.; Hegazi, E. Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ. Sci. Pollut. Res. 2020, 27, 19108–19114. [Google Scholar]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Vašák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta 1985, 827, 36–44. [Google Scholar] [CrossRef]





| GIT Parts | Segment | pH Value | Duration of Transit Time (min) |
|---|---|---|---|
| Foregut | Crop, proventriculus, and gizzard | 3.0 | 90 |
| Middle-gut | Small intestine | 6.2 | 60 |
| Hindgut | Caecum and colon | 5.8 | 30 |
| Item | Starter (Day 1 to 21) | Finisher (Day 22 to 35) |
|---|---|---|
| Ingredient composition (%) | ||
| Corn | 52.5 | 58.5 |
| Soybean meal (45% crude protein) | 37.65 | 31.0 |
| Wheat pollard | 1.35 | 1.00 |
| Palm oil (Refine) | 5.00 | 6.00 |
| Dicalcium phosphate 1 | 1.60 | 1.85 |
| Calcium carbonate | 0.60 | 0.35 |
| Salt (NaCl) | 0.30 | 0.30 |
| DL-Methionine (99%) | 0.25 | 0.25 |
| L-Lysine (78.5% ) | 0.25 | 0.25 |
| Mineral premix 2 | 0.15 | 0.15 |
| Vitamin premix 3 | 0.10 | 0.10 |
| Choline chloride | 0.10 | 0.10 |
| Toxin binder 4 | 0.15 | 0.15 |
| Calculated nutrient composition (% DM, unless stated otherwise) | ||
| Metabolizable energy (Kcal/kg) | 3008 | 3167 |
| Crude protein | 22.6 | 20.09 |
| Crude fat | 7.57 | 8.004 |
| Calcium | 0.9 | 0.76 |
| Available phosphorus | 0.45 | 0.38 |
| Methionine | 0.5 | 0.43 |
| Lysine | 1.32 | 1.05 |
| Threonine | 0.919 | 0.783 |
| Na | 0.23 | 0.23 |
| Analyzed Zn (mg/kg) 5 | 20.83 | 23.33 |
| Items | Treatment Groups 1 | SE 2 | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |||
| Serum (mg/L) | 3.13 ± 0.36 a | 2.09 ± 0.23 a | 3.16 ± 0.05 a | 6.05 ± 0.65 b | 7.74 ± 1.43 b | 0.593 | <0.0001 |
| Liver (mg/kg DM) | 612.5 ± 28.8 a | 400.0 ± 27.0 a | 479.2 ± 25.2 a | 935.8 ± 24.3 a | 1771.7 ± 577.1 b | 211.6 | 0.0004 |
| Tibia (mg/kg DM) | 479.2 ± 15.3 a | 551.7 ± 16.3 ab | 658.3 ± 38.3 b | 663.3 ± 64.9 b | 871.7 ± 118.2 c | 51.84 | 0.0002 |
| Breast (mg/kg DM) | 77.5 ± 9.0 abc | 45.8 ± 26.0 a | 90.8 ± 15.9 ac | 110.0 ± 27.8 c | 172.5 ± 15.0 d | 16.37 | 0.0002 |
| Excreta (g/kg DM) | |||||||
| Day 21 | 7.03 ± 0.04 a | 2.94 ± 0.34 d | 2.97 ± 0.11 d | 3.94 ± 0.15 c | 4.78 ± 0.24 b | 0.167 | <0.0001 |
| Day 35 | 7.08 ± 0.11 a | 2.71 ± 0.15 e | 3.77 ± 0.05 d | 4.14 ± 0.03 c | 4.57 ± 0.12 b | 0.083 | <0.0001 |
| Variable | Treatment Group 1 | SE 2 | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |||
| Serum | |||||||
| SOD 4 (U/mL) | 1.30 ± 0.11 a | 1.63 ± 0.19 a | 2.65 ± 0.05 b | 2.65 ± 0.11 b | 2.57 ± 0.12 b | 0.102 | <0.0001 |
| CAT 5 (U/L) | 5.12 ± 2.13 a | 3.91 ± 2.09 a | 7.64 ± 3.65 a | 13.90 ± 3.55 b | 17.33 ± 0.53 b | 2.162 | 0.0004 |
| TBARS/MDA (µM)6 | 3.56 ± 0.29 a | 4.07 ± 0.23 a | 3.90 ± 0.36 a | 3.74 ± 0.30 a | 3.36 ± 0.05 a | 0.219 | 0.0801 |
| Liver | |||||||
| SOD 4 (U/mL) | 1.33 ± 0.30 a | 1.22 ± 0.38 a | 2.38 ± 0.19 b | 2.21 ± 0.33 b | 2.28 ± 0.07 b | 0.225 | 0.0007 |
| CAT 5 (U/L) | 0.62 ± 0.17 a | 0.38 ± 0.10 a | 0.64 ± 0.08 a | 1.18 ± 0.29 b | 1.82 ± 0.31 c | 0.1728 | <0.0001 |
| TBARS/MDA (µM) 6 | 6.83 ± 0.22 a | 7.36 ± 0.02 a | 7.31 ± 0.50 a | 7.13 ± 0.08 a | 6.76 ± 0.05 a | 0.203 | 0.0609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status. Animals 2023, 13, 115. https://doi.org/10.3390/ani13010115
Mohd Yusof H, Abdul Rahman N, Mohamad R, Zaidan UH, Samsudin AA. Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status. Animals. 2023; 13(1):115. https://doi.org/10.3390/ani13010115
Chicago/Turabian StyleMohd Yusof, Hidayat, Nor’Aini Abdul Rahman, Rosfarizan Mohamad, Uswatun Hasanah Zaidan, and Anjas Asmara Samsudin. 2023. "Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status" Animals 13, no. 1: 115. https://doi.org/10.3390/ani13010115
APA StyleMohd Yusof, H., Abdul Rahman, N., Mohamad, R., Zaidan, U. H., & Samsudin, A. A. (2023). Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status. Animals, 13(1), 115. https://doi.org/10.3390/ani13010115