Emphasizing the Operational Role of a Novel Graphene-Based Ink into High Performance Ternary Organic Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials’ Synthetic Procedures
2.3. OSC Device Fabrication
2.4. Characterization Techniques
3. Results and Discussions
3.1. ATR FT-IR Spectroscopy
3.2. UV-Visible Measurements
3.3. Raman Spectroscopy
3.4. Photoluminescence (PL) Spectroscopy
3.5. XRD Measurements
3.6. Thermogravimetric (TGA) Analysis
3.7. Cyclic Voltammetry Measurements
3.8. Microscopic Characterization
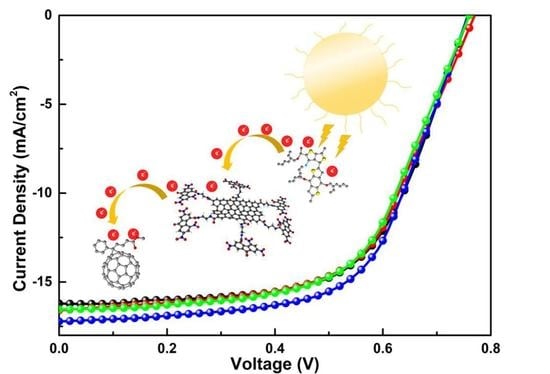
3.9. Photovoltaic Performance Evaluation
3.10. Morphology Characterization of the Active Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Inganäs, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, S.; Kim, G.U.; Lee, W.; Kim, B.J. Recent Advances, Design Guidelines, and Prospects of All-Polymer Solar Cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material Insights and Challenges for Non-Fullerene Organic Solar Cells Based on Small Molecular Acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous Enhancement of Open-Circuit Voltage, Short-Circuit Current Density, and Fill Factor in Polymer Solar Cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2013, 21, 1–11. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photon. 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and Solution-Processed Tandem Solar Cells with 17.3% Efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.L.; Lau, T.K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Liu, T.; Luo, Z.; Chen, Y.; Yang, T.; Xiao, Y.; Zhang, G.; Ma, R.; Lu, X.; Zhan, C.; Zhang, M.; et al. A Nonfullerene Acceptor with a 1000 Nm Absorption Edge Enables Ternary Organic Solar Cells with Improved Optical and Morphological Properties and Efficiencies over 15%. Energy Environ. Sci. 2019, 12, 2529–2536. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Y.; Yao, Y.; Liang, Z. Ternary Blend Strategy for Achieving High-Efficiency Organic Solar Cells with Nonfullerene Acceptors Involved. Adv. Funct. Mater. 2018, 28, 1–20. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Konios, D.; Petridis, C.; Kakavelakis, G.; Stratakis, E.; Kymakis, E. Ternary Solution-Processed Organic Solar Cells Incorporating 2D Materials. 2D Mater. 2017, 4, 042005. [Google Scholar] [CrossRef]
- Gasparini, N.; Salleo, A.; McCulloch, I.; Baran, D. The Role of the Third Component in Ternary Organic Solar Cells. Nat. Rev. Mater. 2019, 4, 229–242. [Google Scholar] [CrossRef]
- Song, X.; Gasparini, N.; Nahid, M.M.; Paleti, S.H.K.; Wang, J.L.; Ade, H.; Baran, D. Dual Sensitizer and Processing-Aid Behavior of Donor Enables Efficient Ternary Organic Solar Cells. Joule 2019, 3, 846–857. [Google Scholar] [CrossRef] [Green Version]
- Seco, C.R.; Vidal-Ferran, A.; Misra, R.; Sharma, G.D.; Palomares, E. Efficient Non-Polymeric Heterojunctions in Ternary Organic Solar Cells. ACS Appl. Energy Mater. 2018, 1, 4203–4210. [Google Scholar] [CrossRef]
- Qin, R.; Guo, D.; Li, M.; Li, G.; Bo, Z.; Wu, J. Perylene Monoimide Dimers Enhance Ternary Organic Solar Cells Efficiency by Induced D-A Crystallinity. ACS Appl. Energy Mater. 2019, 2, 305–311. [Google Scholar] [CrossRef]
- Fu, H.; Li, C.; Bi, P.; Hao, X.; Liu, F.; Li, Y.; Wang, Z.; Sun, Y. Efficient Ternary Organic Solar Cells Enabled by the Integration of Nonfullerene and Fullerene Acceptors with a Broad Composition Tolerance. Adv. Funct. Mater. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Cheng, P.; Li, Y.; Zhan, X. Efficient Ternary Blend Polymer Solar Cells with Indene-C60 Bisadduct as an Electron-Cascade Acceptor. Energy Environ. Sci. 2014, 7, 2005–2011. [Google Scholar] [CrossRef]
- Lu, L.; Xu, T.; Chen, W.; Landry, E.S.; Lu, L. Ternary blend polymer solar cells with enhanced power conversion efficiency. Nat. Photon. 2014, 8, 716–722. [Google Scholar] [CrossRef]
- Lu, L.; Chen, W.; Xu, T.; Yu, L. High-Performance Ternary Blend Polymer Solar Cells Involving Both Energy Transfer and Hole Relay Processes. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Gasparini, N.; Lucera, L.; Salvador, M.; Prosa, M.; Spyropoulos, G.D.; Kubis, P.; Egelhaaf, H.J.; Brabec, C.J.; Ameri, T. High-Performance Ternary Organic Solar Cells with Thick Active Layer Exceeding 11% Efficiency. Energy Environ. Sci. 2017, 10, 885–892. [Google Scholar] [CrossRef]
- Ke, L.; Gasparini, N.; Min, J.; Zhang, H.; Adam, M.; Rechberger, S.; Forberich, K.; Zhang, C.; Spiecker, E.; Tykwinski, R.R.; et al. Panchromatic Ternary/Quaternary Polymer/Fullerene BHJ Solar Cells Based on Novel Silicon Naphthalocyanine and Silicon Phthalocyanine Dye Sensitizers. J. Mater. Chem. A 2017, 5, 2550–2562. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Konios, D.; Viskadouros, G.; Vernardou, D.; Katsarakis, N.; Koudoumas, E.; Anastasiadis, S.H.; Stratakis, E.; Kymakis, E. Ternary Organic Solar Cells Incorporating Zinc Phthalocyanine with Improved Performance Exceeding 8.5%. Dyes Pigm. 2017, 146, 408–413. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Balis, N.; Stylianakis, M.M.; Savarese, M.; Adamo, C.; Gemmi, M.; Pellegrini, V.; Stratakis, E.; Kymakis, E. Functionalized Graphene as an Electron-Cascade Acceptor for Air-Processed Organic Ternary Solar Cells. Adv. Funct. Mater. 2015, 25, 3870–3880. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Konios, D.; Kakavelakis, G.; Charalambidis, G.; Stratakis, E.; Coutsolelos, A.G.; Kymakis, E.; Anastasiadis, S.H. Efficient Ternary Organic Photovoltaics Incorporating a Graphene-Based Porphyrin Molecule as a Universal Electron Cascade Material. Nanoscale 2015, 7, 17827–17835. [Google Scholar] [CrossRef] [PubMed]
- Sygletou, M.; Tzourmpakis, P.; Petridis, C.; Konios, D.; Fotakis, C.; Kymakis, E.; Stratakis, E. Laser Induced Nucleation of Plasmonic Nanoparticles on Two-Dimensional Nanosheets for Organic Photovoltaics. J. Mater. Chem. A 2016, 4, 1020–1027. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Del Rio Castillo, A.E.; Pellegrini, V.; Ansaldo, A.; Tzourmpakis, P.; Brescia, R.; Prato, M.; Stratakis, E.; Kymakis, E.; Bonaccorso, F. Size-Tuning of WSe2 Flakes for High Efficiency Inverted Organic Solar Cells. ACS Nano 2017, 11, 3517–3531. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Viskadouros, G.; Polyzoidis, C.; Veisakis, G.; Kenanakis, G.; Kornilios, N.; Petridis, K.; Kymakis, E. Updating the Role of Reduced Graphene Oxide Ink on Field Emission Devices in Synergy with Charge Transfer Materials. Nanomaterials 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Nagarjuna, P.; Bagui, A.; Gupta, V.; Singh, S.P. A Highly Efficient PTB7-Th Polymer Donor Bulk Hetero-Junction Solar Cell with Increased Open Circuit Voltage Using Fullerene Acceptor CN-PC70BM. Org. Electron. 2017, 43, 262–267. [Google Scholar] [CrossRef]
- Fan, R.; Huai, Z.; Sun, Y.; Li, X.; Fu, G.; Huang, S.; Wang, L.; Yang, S. Enhanced Performance of Polymer Solar Cells Based on PTB7-Th:PC 71 BM by Doping with 1-Bromo-4-Nitrobenzene. J. Mater. Chem. C 2017, 5, 10985–10990. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Z.; Li, W.; Yu, Z.Z.; Rafiee, M.A.; Rafiee, J.; Yavari, F.; Koratkar, N. Enhanced thermal stability in graphene oxide covalently functionalized with 2-amino-4, 6-didodecylamino-1, 3, 5-triazine. Carbon 2011, 49, 1258–1265. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Azimi, H.; Senes, A.; Scharber, M.C.; Hingerl, K.; Brabec, C.J. Charge Transport and Recombination in Low-Bandgap Bulk Heterojunction Solar Cell using Bis-adduct Fullerene. Adv. Energy Mater. 2011, 1, 1162–1168. [Google Scholar] [CrossRef]
- Lu, L.; Kelly, M.; You, W.; Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photon. 2015, 9, 491–500. [Google Scholar] [CrossRef]











| GO-TNF Content (%) | Jsc (mA/cm2) | Calc. Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| 0 | 16.20 ± 0.45 | 15.72 | 0.760 ± 0.010 | 61.8 ± 0.7 | 7.61 ± 0.11 |
| 1 | 16.54 ± 0.54 | 16.21 | 0.760 ± 0.005 | 63.0 ± 0.4 | 7.92 ± 0.26 |
| 2 | 17.21 ± 0.44 | 16.78 | 0.760 ± 0.011 | 64.0 ± 0.1 | 8.37 ± 0.34 |
| 3 | 16.53 ± 0.35 | 16.21 | 0.760 ± 0.009 | 62.4 ± 0.6 | 7.84 ± 0.17 |
| Active Layer | μh (cm2 V−1 s−1) | μe (cm2 V−1 s−1) | Ratio (μh/μe) |
|---|---|---|---|
| PTB7:PC71BM (reference) | 1.28 × 10−4 | 7.80 × 10−5 | 1.64 |
| 1% GO-TNF | 1.31 × 10−4 | 8.71 × 10−5 | 1.50 |
| 2% GO-TNF | 1.39 × 10−4 | 9.93 × 10−5 | 1.39 |
| 3% GO-TNF | 1.34 × 10−4 | 8.03 × 10−5 | 1.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianakis, M.M.; Kosmidis, D.M.; Anagnostou, K.; Polyzoidis, C.; Krassas, M.; Kenanakis, G.; Viskadouros, G.; Kornilios, N.; Petridis, K.; Kymakis, E. Emphasizing the Operational Role of a Novel Graphene-Based Ink into High Performance Ternary Organic Solar Cells. Nanomaterials 2020, 10, 89. https://doi.org/10.3390/nano10010089
Stylianakis MM, Kosmidis DM, Anagnostou K, Polyzoidis C, Krassas M, Kenanakis G, Viskadouros G, Kornilios N, Petridis K, Kymakis E. Emphasizing the Operational Role of a Novel Graphene-Based Ink into High Performance Ternary Organic Solar Cells. Nanomaterials. 2020; 10(1):89. https://doi.org/10.3390/nano10010089
Chicago/Turabian StyleStylianakis, Minas M., Dimitrios M. Kosmidis, Katerina Anagnostou, Christos Polyzoidis, Miron Krassas, George Kenanakis, George Viskadouros, Nikolaos Kornilios, Konstantinos Petridis, and Emmanuel Kymakis. 2020. "Emphasizing the Operational Role of a Novel Graphene-Based Ink into High Performance Ternary Organic Solar Cells" Nanomaterials 10, no. 1: 89. https://doi.org/10.3390/nano10010089
APA StyleStylianakis, M. M., Kosmidis, D. M., Anagnostou, K., Polyzoidis, C., Krassas, M., Kenanakis, G., Viskadouros, G., Kornilios, N., Petridis, K., & Kymakis, E. (2020). Emphasizing the Operational Role of a Novel Graphene-Based Ink into High Performance Ternary Organic Solar Cells. Nanomaterials, 10(1), 89. https://doi.org/10.3390/nano10010089